Abstract
Fatty acid metabolism, particularly fatty acid synthesis, is a very important cellular physiological process in which nutrients are used for energy storage and biofilm synthesis. As a key enzyme in the fatty acid metabolism, fatty acid synthase (FASN) is receiving increasing attention. Although previous studies on FASN have mainly focused on various malignancies, many studies have recently reported that FASN regulates the survival, differentiation, and function of various immune cells, and subsequently participates in the occurrence and development of immune-related diseases. However, few studies to date systematically summarized the function and molecular mechanisms of FASN in immune cell biology and related diseases. In this review, we discuss the regulatory effect of FASN on immune cells, and the progress in research on the implications of FASN in immune-related diseases. Understanding the function of FASN in immune cell biology and related diseases can offer insights into novel treatment strategies for clinical diseases.
Similar content being viewed by others
Facts
-
FASN, a key enzyme in fatty acid metabolism, can regulate the survival, differentiation and function of various immune cells.
-
Dysregulation of FASN contributes to the occurrence and development of immune-related diseases.
-
Targeting FASN has gained considerable attention as a promising therapeutic approach for immune-related diseases.
Open Questions
-
What factors are involved in regulating the expression of FASN in different immune microenvironments?
-
What is the regulatory effect of FASN on immune cells and its mechanism?
-
What is the role of FASN in the pathogenesis of different types of immune-associated diseases?
-
How does targeted inhibition of FASN affect the incidence of immune-associated diseases?
Introduction
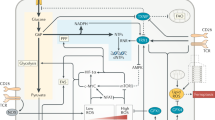
Fatty Acid Synthetase (FASN) is an essential enzyme in the de novo synthesis of endogenous long-chain fatty acids [1]. During the catalysis of FASN, acetyl CoA and malonyl CoA are used as raw materials, and the main products include palmitic acid (80%), stearic acid (10%), and myristic acid (10%) [2]. As a macromolecular multifunctional polymerase, FASN comprises seven catalytic domains, including condensation, transacylation, reduction, and dehydration [3]. In humans, the gene encoding FASN is located on chromosome 17q25; it is approximately 20 kb long, and contains 43 exons, 42 introns, 3 initiation sites, and 3 promoters. In mammals, FASN exists and functions as a dimer, with two identical peptide chains, and a molecular weight of approximately 260 kDa each. Once the dimer is depolymerized, it loses its activity [3]. The N-terminal region of FASN contains three catalytic domains (ketoacyl-synthase, dehydrase, and monoacyl/acetyltransferase), and the C-terminal region contains four domains (alcohol reductase, ketoacyl-reductase, acyl carrier protein, and thioesterase), with a core region comprising 600 amino acid residues in the middle (Fig. 1).
a The location of the FASN gene. b Generalized linear domain map of animal FASN. c The basic structure of the human FASN protein 3D structure (http://www.genecards.org). d FASN-mediated biosynthesis pathway of fatty acids. Glucose is phosphorylated to glucose-6P when ingested by cells and enters the glycolysis pathway. The resulting pyruvate enters the mitochondria and is converted to acetyl-CoA before entering the citric acid cycle. The citrate then travels through the mitochondria and is converted to acetyl-CoA by the ACL. Then, acetyl-CoA is converted to malonyl CoA. Using NADPH as a reduction cofactor, FASN catalyzes the synthesis of acetyl-CoA and malonyl-CoA palmitate and then synthesizes complex fatty acid metabolites. TCA tricarboxylic acid cycle, NADPH nicotinamide adenine dinucleotide phosphate hydrogen.
Under physiological conditions, human tissues mainly use exogenous fatty acids, and the synthesis level of endogenous fatty acids is low. In some pathological conditions such as tumors [4, 5], cardiovascular diseases [6, 7], inflammatory diseases [8,9,10,11], and autoimmune diseases [12, 13], FASN expression and the content of fatty acid products synthesized from FASN are abnormally changed. Since numerous studies have corroborated the vital role of FASN-mediated fatty acid synthesis in supporting cellular activities and its involvement in diverse biological processes [14], FASN has been identified as a significant contributor to the progression of numerous diseases. Consequently, targeting FASN has gained considerable attention as a promising therapeutic approach for these conditions, thus emerging as a prominent area of research interest [2].
This review provides an overview of our current understanding of the molecular mechanism of FASN expression regulation. Furthermore, we highlight the implications of FASN in immune cell biology and related diseases. By understanding these processes, future therapeutic strategies could be shaped to develop new strategies for treating immune-related diseases.
Regulation of FASN expression and activity
Transcription factor SREBP
Sterol regulatory element-binding proteins (SREBPs) are crucial nuclear transcription factors that participate in lipid metabolism in eukaryotic cells [15]. Extensive research has substantiated that SREBPs play a significant role in promoting the expression of FASN by recognizing binding sites at the proximal promoter of FASN [16]. Although SREBPs consist of three subtypes, SREBP-1a, SREBP-1c and SREBP-2, it has been discovered that only SREBP-1a and SREBP-1c can activate the transcription of genes involved in fatty acid and triglyceride synthesis, including FASN. In a normal physiological state, SREBPs exist in an inactive form (named pSREBP), mainly on the surface of the endoplasmic reticulum and nuclear membrane. However, under certain pathological conditions or when lipid levels are low, SREBP undergoes sequential cleavage, leading to the formation of mature SREBP. Mature SREBP is then transported into the nucleus, and promotes the transcription of various target genes implicated in adipogenesis and cholesterol biosynthesis [17].
Regulation of the SREBP-FASN axis is involved in different biological processes. Many proteins, including membrane-bound transcription factor protease site 2 (MBTPS2), CD36, spindle protein 1 (SPIN1), and so on, can promote the expression of FASN by enhancing the expression of SREBP, interacting with SREBPs, or promoting the nuclear translocation of SREBPs to participate in the regulation of biological processes mediated by fatty acid metabolism [18,19,20,21,22,23]. For example, nuclear factor Y (NF-Y) activates the transcription of SREBP-1 by directly binding to the CCAAT motif in the SREBP-1 promoter and subsequently leads to enhanced expression of SREBP-1 and FASN, consequently contributing to the progression of alcoholic liver disease [24]. Targeted inhibition of SREBPs can consistently inhibit FASN expression and FASN-mediated fatty acid synthesis [25,26,27,28]. For instance, in high-fat diet-induced hyperlipidemic rats, the combined supplementation of selenium and vitamin B6 inhibits FASN expression by blocking the SIRT1/SREBP-1c signaling axis, and this intervention demonstrates an improvement in dyslipidemia and fatty liver syndrome [29]. In conclusion, all these studies indicate that SREBPs can indeed regulate the expression of FASN (Fig. 2a and Table 1).
a SREBPs play a significant role in controlling the expression of FASN by recognizing binding sites at the proximal promoter of FASN. Endogenous proteins and exogenous substances can promote or inhibit SREBP-mediated FASN transcription. b The PI3K-AKT signaling pathway participates in regulating the SREBP-FASN signal axis. Endogenous proteins and exogenous substances can regulate FASN expression by triggering or inhibiting the PI3K-AKT signaling pathway. c Noncoding RNAs can regulate FASN expression by targeting FASN or SREBPs. d Some proteins can directly regulate the stability of FASN at the mRNA or protein level. MBTPS2 membrane-bound transcription factor protease site 2, SPIN1 spindle protein 1, NF-Y nuclear factor Y, NS5A nonstructural protein 5A, SIK2 salt inducible kinase 2, NUPR1 nuclear protein 1, HIF1 hypoxia-inducible factor-1, YAP Yes-associated protein, LAMP3 lysosomal associated membrane protein 3, IGF-1R insulin-like growth factor 1 receptor, PTEN phosphatase and tensin homolog deleted on chromosome ten, AZGP1 Zinc-alpha-2-glycoprotein, Eif6 eukaryotic initiation factor 6, USP38 ubiquitin carboxyl-terminal hydrolase 38, PHLPP1 PH domain leucine-rich repeat protein phosphatase 1, ROS reactive oxygen species, GNPAT glyceronephosphate Oacyltransferase.
PI3K-AKT signaling pathway
Phosphatidylinositol kinase PI3K is a very important phosphatidylinositol 3-kinase that produces the second messenger phosphatidylinositol 3,4,5-triphosphate (PI-3,4,5-P3). As a critical regulatory molecule for cellular metabolism and growth, PI3K governs essential biological processes such as nutrient uptake, energy generation, cofactor production, and macromolecular biosynthesis [30]. Consequently, PI3K can be regarded as the central driving force behind cellular metabolism.
There is substantial evidence that the PI3K-AKT signaling pathway participates in many biological processes by regulating the SREBP-FASN signaling axis [31,32,33,34,35,36,37]. For example, PTEN, a protein phospholipid phosphatase, can target PIP3 to block AKT activation, thereby inhibiting FASN expression [38]. In addition, pharmacological inhibition of the PI3K-AKT pathway can effectively impede FASN activity or expression, and its associated fatty acid synthesis [39,40,41]. For instance, orlistat can inhibit FASN activity by regulating the AKT pathway, thereby exerting its anti-lipogenesis and anti-proliferative effects [42]. In conclusion, the PI3K-AKT pathway has a critical impact on the regulation of FASN expression and thereby influences multiple biological processes (Fig. 2b and Table 1).
Noncoding RNA (ncRNA)
Noncoding RNAs, including microRNAs, long-chain noncoding RNAs, and circulating RNAs, have been shown to widely participate in various networks in which they can modulate complex molecular and cellular processes [43, 44]. Notably, it has been shown that noncoding RNAs can directly or indirectly regulate FASN expression and modulate fatty acid synthesis.
At present, the regulation of miRNA on the expression of FASN has been widely studied. On the one hand, some miRNAs, including miR-497-5p, miR-328-5p, miR-103, and miR-4310, can directly target FASN and inhibit its expression [45,46,47,48]. On the other hand, some miRNAs can regulate FASN expression indirectly. For example, miR-33b can target the transformation of growth factor β-activated kinase 1 (TAK1), leading to the inhibition of FASN activity and modulation of lipid metabolism [49]. Another miRNA, miRNA-212, regulates FASN expression by specifically targeting SIRT2 [50]. However, to date, there are few studies on the regulation of FASN expression by long noncoding RNAs and circulating RNAs. Small nucleolar RNA host gene 25 (SNHG25), a long-strand ncRNA, regulates FASN expression through miR-497–5p [51]. Some circRNAs can also regulate FASN expression and participate in multiple biological processes [52,53,54].
Notably, some noncoding RNAs can indirectly regulate FASN expression and modulate fatty acid synthesis by influencing the expression or activity of SREBPs [55,56,57]. For instance, the long noncoding RNA ZFAS1 interacts with polyadenylate binding protein 2 (PABP2), promoting the stabilization of SREBP-1 mRNA. Consequently, this interaction elevates the expression of SREBP-1 and its downstream target gene FASN, thereby promoting lipid accumulation in colorectal cancer (CRC) [58]. In conclusion, noncoding RNAs are also involved in regulating the expression of FASN (Fig. 2c and Table 1).
Other proteins
In addition to the above three key molecules or signaling pathways involved in regulating FASN expression, several other proteins have also been found to be involved in regulating FASN expression, including eukaryotic initiation factor 6 (Eif6), the ubiquitin-specific protease USP38, pleckstrin homology domain leucine-rich repeat protein phosphatase 1 (PHLPP1), and glycerophospho-o-acyltransferase (GNPAT) [59,60,61,62]. These molecules can affect the stability of FASN at the mRNA or protein level and thus participate in various biological processes (Fig. 2d and Table 1).
FASN and immune cells
Macrophages
Macrophages, which are an important component of the innate immune system, exhibit distinct activation states and diverse characteristics and functions influenced by alterations in the tissue microenvironment. Over the past years, multiple investigations have demonstrated the significant involvement of macrophage polarization, primarily categorized as M1 and M2, in various pathophysiological processes, including inflammation, tumor development, tissue repair, and metabolism. M1 macrophages can promote inflammation, eliminate pathogenic microorganisms, and show antitumor effects; M2 macrophages exhibit regulatory functions, such as inhibiting inflammation and promoting tissue remodeling [63, 64].
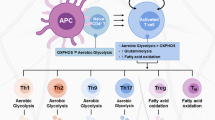
It has been demonstrated that FASN and FASN-mediated fatty acid synthesis play a crucial regulatory role in the polarization of M1 macrophages. The polarization of M0 macrophages to M1 macrophages is accompanied by an enhanced level of FASN expression and significant changes in FASN-mediated fatty acid metabolism [9]; high expression of FASN can significantly promote M1-type macrophage activation, while the inhibition or deletion of FASN can significantly reduce M1-type macrophage activation [9, 65]. For instance, the deletion of FASN, particularly its ketoacyl synthase domain, impairs M1 macrophage activation by reducing acetoacetyl-CoA levels and thereby obstructing cholesterol synthesis [65]; FASN deficiency results in impaired cholesterol retention in the plasma membrane and disrupts Rho GTPase trafficking and JNK activation, which are major mediators of M0 to M1 macrophage transformation [66]. Additionally, FASN can also contribute to the pro-inflammatory role of macrophages by promoting Akt palmitoylation, which further enhances the activation of Akt-MAPK signaling [9]. These studies suggest that FASN plays a crucial role in the polarization and activation of M1-type macrophages (Fig. 3).
Moreover, FASN is also involved in regulating NLRP-3-mediated caspase-1 activation, pro-1β expression, and reactive oxygen species (ROS) production in macrophages [67]. Furthermore, the FASN protein can promote the formation of ox-LDL-induced macrophage foam cells, thereby augment the activation of macrophages and contribute to the pathogenesis of atherosclerosis [68]. However, few studies have been conducted on regulating M2 macrophage polarization by FASN. Thus far, only one study has shown that circ_0018909 induces the polarization of M0 macrophages to M2 macrophages by regulating the signaling axis of miR-545–3p-FASN and then participates in the pathogenesis of pancreatic cancer [54]. It follows that FASN is widely involved in regulating M1-type macrophage polarization.
In addition, FASN is also involved in regulating the function and chemotaxis of macrophages. HIF-1α deletion promotes lipid formation and lipid accumulation in macrophages through the mTOR-SREBP-1c-FASN axis; thus, myeloid cells from myelo-restricted HIF-1α-deficient mice and individuals with HIF1A gene polymorphism dysfunction are more likely to be infected with Leishmania donovani through increased lipogenesis, thereby suggesting that FASN-mediated lipogenesis and lipid accumulation are involved in the regulation of the bactericidal function of macrophages [21]. FASN can also contribute to the pro-inflammatory role of macrophages by promote Akt palmitoylation, which further enhance the activation of Akt-MAPK signaling [9]. Furthermore, FASN can also regulate the recruitment and infiltration of macrophages [69, 70]. For instance, FASN deficiency alters membrane order and composition, impairing the retention of plasma membrane cholesterol and disrupting Rho GTPase trafficking-a process needed for cell adhesion, migration and activation. This disruption leads to reduced recruitment of macrophages to adipose tissue and chronic inflammation in mice, preventing diet-induced insulin resistance [66]. Also, LPS can trigger macrophage chemotaxis via the SREBP-1c/FASN/palmetto signaling pathway, inhibition of FASN expression significantly alleviated LPS-augmented CCL2 secretion, indicating that FASN can contribute to the recruitment and progression of macrophages in periapical inflammation [69]. Together, considering the regulatory effect of FASN on macrophages, FASN may become a therapeutic direction for macrophage-mediated inflammatory diseases (Fig. 3).
T lymphocytes
T cells, which mainly mediate adaptive immunity, are composed of a group of heterogeneous lymphocytes with different functions. CD8+ T cells play an important role in the immune system’s defense against pathogens and tumors [71], while CD4+ T cells, including subpopulations such as helper T cells 1 (Th1), Th17, regulatory T cells (Tregs) and so on, mainly involved in antimicrobial immunity, autoimmunity, and tumor immunity [72, 73].
Notably, the activation and differentiation of T cells are often accompanied by significant changes in fatty acid metabolism, and FASN-mediated lipid metabolism can widely affect the survival, differentiation, and function of T cells. Notably, one research has shown that FASN is a key metabolic control that generates the inflammatory subgroups of Th17 cells. The activation of Th17 cells by IL-23 and Pam3CSK4, an agonist of TLR2/1, can lead to the increased expression of FASN, subsequently contributing to the survival and proliferation of Th17 cells; the inhibition of FASN, particularly in Th17 cells, significantly attenuated the disease in a mouse model of experimental autoimmune encephalomyelitis; Conversely, the inhibition of FASN function promotes the production of IFN-γ by Th1 and Th1-like Th17 cells [12]. However, the molecular mechanism by which FASN plays a unique role in promoting Th17 differentiation remains to be determined. It is well known that Th17 is differentiated by Th0 cells under the induction of IL-6 and IL-23, which activate the JAK-STAT3 signaling pathway and induce the expression of important transcription factors, such as RORγ and IL17 along with other pro-inflammatory factors [74]. Since targeting FASN had no effect on the expression of other lineage-specific transcription factors, like Foxp3, GATA binding protein 3, and T-bet, FASN may directly or indirectly regulate the activation of JAK-STAT3 signaling pathway or the transcription of the Th17 genetic program. Therefore, future experiments will be necessary to determine the mechanism underlying the regulation of Th17 function by FASN (Fig. 3).
In addition, FASN has been proven to be related to restimulation-induced cell death (RICD). RICD is a type of cell death triggered by T cell receptor (TCR) reinvolvement in activating effector T cells to ensure that the expansion of the T-cell population is maintained in an inhibited state. Blocking FASN with compound C75 can markedly protect CD4+ T cells from the effects of RICD, thus suggesting that FASN can promote sensitivity to RICD (Fig. 3) [75].
Regulatory T cells (Tregs) possess immunosuppressive functions and can maintain immune homeostasis, suppress inflammation, and promote cancer [76]. The advantage of Tregs in the TME is closely tied to energy supply pathways associated with lipid metabolism. Notably, FASN-mediated fatty acid synthesis is crucial for the functional maturation of Treg cells. Despite normal TCR-induced proliferation, FASN deletion impaired the TCR-dependent upregulation of Treg activation and maturation markers, including GITR and CD44; moreover, de novo fatty acid synthesis mediated by FASN contributes to the functional maturation of Treg cells, and the loss of FASN from Treg cells inhibits tumor growth, which may be related to dysregulated activation of PI3K in intratumoral Treg cells [5]. These data collectively indicate that FASN contributes to the functional maturation of Treg cells, and inhibiting FASN-dependent lipid synthesis, and the release of metabolic signals from Tregs effectively suppresses the antitumor immune response (Fig. 3) [5]. Another study also found that the upregulation of FASN expression in Tregs of APS-1, an organ-specific autoimmune disease characterized by single-gene mode inheritance, indicates increased metabolic activity and correlates with functional impairments of Tregs [13]. However, the exact mechanism remains unclear. Taken together, the above studies confirm that FASN-mediated metabolic reprogramming enhances the functional specialization of Treg cells in tumors and provides a novel approach for the targeted therapy of Treg cells in tumors.
Moreover, FASN is also involved in regulating the infiltration and activation of T cells. One study showed that the PI3k-α-specific inhibitor CYH33 facilitates the infiltration and activation of T cells by promoting FASN-mediated fatty acid metabolism while weakening the proliferation of M2-like macrophages and Treg cells; this boosts host immunity and inhibits tumor growth [77]. Similarly, another study also reported that FASN expression in gastric cancer tissues was closely related to the levels of immune infiltration of T cells [70]. In conclusion, FASN can regulate the infiltration and activation of T cells (Fig. 3).
Other cells
As is known, dendritic cells (DCs) play an important role in immune responses by activating naïve T cells [78]. According to previous studies, the synthesis of fatty acids is essential for the maturation and function of DCs. Inhibition of FASN reduced the proportion of CD11c+ cells in mouse liver, bone marrow and spleen and improved the ability of DCs to capture antigens [79]. Similarly, FASN is highly expressed in ovarian cancer and can inhibit the capacity of tumor-infiltrating DCs (TIDCs), thereby reducing the ability of T cells to fight tumors (Fig. 3) [80].
Neutrophils, the most important innate immune cells against pathogenic microorganisms, play a promoting role in the pathogenesis of sepsis. The Toll-like receptor (TLR)/myeloid differentiation major reactive protein (MyD88) signaling pathway exacerbates sepsis by affecting the migration of neutrophils to the site of infection. Notably, FASN inhibition by C75 could improve neutrophil chemotaxis and their survival rate in septic mice. C75 specifically blocks the TLR/MyD88 signaling pathway in neutrophils, inhibiting CXCR2 internalization, which in turn promotes the ability of neutrophils to chemotaxis IL-8, thus effectively inhibiting septic inflammation (Fig. 3) [8].
Notably, B cells play a central role in humoral immunity. Germinal centers (GCs) are the sites where B cells differentiate into plasma cells, and produce antibodies; they are therefore essential for humoral immunity [81]. One study has shown that the expression levels of SREBP and FASN in GC B cells are higher than those in naive B cells, and the signaling axis of SREBP-FASN can promote the proliferation of GC B cells through upregulation of lipid homeostasis in vivo (Fig. 3) [82].
FASN and immune-related diseases
Tumors
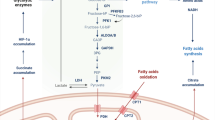
Malignant tumors are a serious risk to people’s health and affect social labor productivity. The immune system has been recognized to play an irreplaceable role in controlling the pathogenesis of tumors. There is increasing evidence that the local tumor microenvironment (TME) has immunosuppressive properties [83]. Previous studies have mainly focused on the function of FASN in the proliferation and metastasis capability of tumor cells [2]. Recently, increasing attention has been given to the effect of abnormal activation of this lipogenesis enzyme on the host antitumor immune environment. It has been shown that FASN and FASN-mediated fatty acid synthesis contribute to the pathogenesis of many different types of tumors by regulating the immunosuppressive properties of the TME. For example, as previously mentioned, the lipid synthesis and metabolic signals controlled by FASN in Treg cells are indispensable for their function and maturation; deleting FASN effectively impairs the function and maturation of Treg cells, thereby resulting in an enhanced antitumor immune response, and inhibition of colorectal cancer development [5].
According to several studies, in 35 different cancers, the higher the level of FASN expression, the less the infiltration of immune cells with antitumor function in the TME [84]. For example, FASN was overexpressed in gastric cancer tissues, and its expression was negatively correlated with the immune cell infiltration levels of CD4+ T cells, CD8+ T cells, macrophages, neutrophils, and DCs [70]. Additionally, in melanoma patients with FASN mutations, the immune cell infiltration levels of initial B cells, CD4+ T cells, cytotoxic cells, effector memory CD4+ T cells and DCs were enhanced. In contrast, those of Treg cells and M2 macrophages were reduced, thereby preventing the metastasis and proliferation of melanoma cells [85]. It needs to be pointed out in particular that M2 macrophages share common markers and mechanisms of action with mononuclear myeloid-derived suppressor cells (M-MDSCs), which are a type of suppressive myeloid antigen-presenting cells that have been shown to promote tumor progression and correlate with poor prognosis in cancer patients. For example, both cell populations express arginase-1 (arg-1), inducible nitric oxide synthase (iNOS), and programmed death ligand 1 (PD-L1), and they secrete immune-suppressive cytokines such as IL-10. M2 macrophages express CD115 and interferon regulatory factor 8 (IRF8) more intensely but not S100A9 [86]. Additionally, it has been shown that tumor infiltrating MDSCs can differentiate into M2 macrophages.
Moreover, the abnormal expression of FASN has been proved to be associated with the sensitivity and prognosis of malignant tumors to immunotherapy, especially PD-1 therapy [84]. As is known, PD-1 can negatively regulate the function of T cells, and contribute to the immune escape of tumor cells. Docosahexaenoic acid (DHA), an omega-3 polyunsaturated fatty acid, promotes the degradation of PD-L1 by inhibiting the expression of FASN, and then reverses the PD-L1-mediated immunosuppression, thus inhibiting tumor growth [87]. Another study has also shown that the upregulation of FASN participates in the resistance of lung cancer cells to natural killer cell-mediated cytotoxicity [88]. These findings indicate that FASN holds potential as an effective prognostic indicator and immunotherapeutic target for diverse types of malignant tumors.
In addition, in the tumor immune microenvironment, cells with immunosuppressive functions, such as Treg cells, can promote tumor immune escape by inhibiting the differentiation and function of some pro-inflammatory immune cells, including M1 macrophages, dendritic cells, Th1 cells, CD8+ T cells and NK cells [89]. For example, M1 macrophages can express a large number of proinflammatory cytokines, including TNF-α, arginase-1 and iNOS, thereby participating in the Th1 cell-mediated anti-tumor immune response, and recruiting CD8+ T cells and NK cells [90]. Since specifically blocking FASN in Treg cells can inhibit the maturation and function of Tregs [5], targeting FASN can eliminate the inhibitory effect of Tregs on the antitumor function of immune cells, leading to the activation of M1 macrophages and CD8+ T cells. Thus, targeting FASN can enhance antitumor immunity, and has the potential to be a unique approach to tumor immunotherapy.
Taken together, FASN can contribute to the TME formation and tumor immune response. On the one hand, FASN participates in tumor immunity by regulating the infiltration of immune cells in the tumor immune microenvironment; on the other hand, FASN participates in tumor immunity by directly affecting the differentiation and function of immune cells (Fig. 4).
Infectious diseases
Infectious diseases, such as coronavirus disease 2019 (COVID-19), are local tissue and systemic inflammatory responses caused by pathogen invasion into the body, which can cause multiple organ damage and death and have become a major global public health challenge [91]. It has been confirmed that FASN is involved in regulating the replication of various viruses [92, 93]. For example, the lipid status of cells regulates the replication and infectivity of hepatitis C virus (HCV), and miR-27a can inhibit HCV replication and infection by inhibiting the expression of FASN and SREBP1 [94]; Infection with Singapore Irivirus (SGIV) increases the expression of key enzymes of fatty acid synthesis in vivo and in vitro, such as FASN and SREBP1, and FASN regulates the replication of SGIV by affecting viral gene transcription and protein expression [95]. Regarding COVID-19, cellular lipid synthesis is a prerequisite for SARS-CoV-2 replication. In vitro experimental results and clinical data show that orlistat, a drug inhibitor of FASN, can markedly block coronavirus replication, and renew immune homeostasis [96]. These findings indicate that FASN is involved in regulating virus infection; therefore, FASN inhibitors have promising potential in treating infectious diseases.
Precisely because lipid metabolism regulates viral replication and the host antiviral immune response, the role of lipid metabolism in viral infection has attracted considerable attention. Notably, type I interferons (IFN-I) can trigger a cascade of signals that ultimately activate hundreds of genes known as interferon stimulator genes (ISGs), which together promote an antiviral state. Interestingly, IFN-I can significantly downregulate the expression of FASN, thereby reducing viral infection [10], which reveals a novel mechanism for the antiviral activity of IFN-I, namely the downregulation of metabolic gene expression. Together, FASN can indeed regulate viral replication and host antiviral immune response (Fig. 4).
Autoimmune diseases
Autoimmune diseases (AIDs) are caused by the abnormal activation of immune cells to imbalance autoimmune homeostasis, and the incidence of these diseases is increasing worldwide [97]. Since FASN has an enormous impact on the activation, differentiation and function of immune cells, it is reasonable to assume that FASN is involved in the occurrence and development of AID. However, to date, few studies have explored the function of FASN in AID.
Multiple sclerosis (MS) is a common inflammatory demyelinating disease, and experimental autoimmune encephalomyelitis (EAE) is a model of MS disease mediated primarily by specifically sensitized CD4+ T cells. FASN has been characterized as a key metabolic control for the production of inflammatory subsets of Th17 cells, and inhibition of FASN, particularly in Th17 cells, can reduce the severity of EAE [12].
Rheumatoid arthritis (RA) is a typical chronic systemic autoimmune disease. Morin, a natural flavonoid, can block Th17 differentiation by restricting FASN transcription and subsequently alleviate collagen-induced arthritis. However, another study showed that Bi Zhong Xiao decoction (BZXD) can promote the expression of FASN, and affect fatty acid metabolism, thereby exerting therapeutic effects on RA [98]. It can be seen that the role of FASN in the pathogenesis of RA is still controversial.
Autoimmune polyendocrine syndrome type I (APS-1) is a single-gene mode disease of organ-specific autoimmunity that is caused by mutations in the autoimmune regulatory factor (AIRE) gene. Compared to healthy subjects, FASN expression in Tregs was increased in APS-1 patients; this may be related to the functional defects of Tregs. Functional studies are needed to determine the implications of these findings for APS-1 immunopathogenesis and Treg immunobiology [13]. These studies suggest that FASN truly has a significant regulatory role in the pathogenesis of AID (Fig. 4).
Inflammatory bowel disease
Inflammatory bowel disease (IBD) is a common disease caused by an overactive immune system that invades the walls of the gastrointestinal tract, thereby causing inflammation and ulcers [99]. It has been shown that FASN contributes to the pathogenesis of IBD: FASN can interact with Hakai and contribute to the occurrence of IBD, suggesting that FASN-mediated intestinal barrier function homeostasis may be one of the important mechanisms in the pathogenesis of IBD [100]. Moreover, the FASN inhibitor C75 can significantly inhibit the expression of FASN and effectively reduce the severity of experimental colitis by inhibiting dextran sodium sulfate (DSS)-induced activation of the inflammatory pathway [101]. Moreover, metformin, a hypoglycemic drug, can improve DSS-induced colitis by blocking the proinflammatory activation of macrophages by inhibiting the FASN/Akt pathway [9]. Thus, FASN participates in the pathogenesis of IBD, and inhibition of FASN may be an effective treatment for IBD (Fig. 4).
Diabetes
Diabetes mellitus (DM) and its precursor, insulin resistance, is a metabolic disease caused by abnormalities in the transfer, transportation, and/or storage of energy substrates. There is increasing evidence that FASN is associated with DM and insulin resistance. Notably, knocking down FASN expression in macrophages significantly prevents diet-induced insulin resistance and leads to recruitment of macrophages to adipose tissue and chronic inflammatory sites in mice [66], indicating that endogenous fat production in macrophages is essential to develop exogenous adipose-induced insulin resistance.
A major cause of end-stage renal disease is diabetic nephropathy (DN). miR-544 can directly target the mRNA of FASN, and subsequently inhibit glomerulosclerosis and inflammation, thereby alleviating diabetic kidney injury [102], suggesting that FASN is involved in the pathogenesis of DN and that targeting FASN could be a potential approach to treat DN. Also, FASN can regulate the transcription and selective splicing levels of immune/inflammation-related genes in islet cells, thus contributing to the immune metabolism of islet cells [103]. These studies provide new insights for interpreting the function of FASN in the immunological mechanisms underlying the onset of DM and insulin resistance (Fig. 4).
Nonalcoholic fatty liver disease
The incidence of nonalcoholic fatty liver disease (NAFLD) has remained high throughout the world, and it has become the most common cause of chronic liver disease. However, to date, there is no recognized drug treatment for NAFLD. FASN has become an attractive therapeutic target for NASH due to its ability to aggravate the development of NAFLD by mediating pro-inflammatory and fibrotic signals. The inhibition of FASN activity could alleviate the disease by reducing Th17 cell production and IL-1β release [104]. This finding suggests that FASN inhibition can reduce the inflammation of NAFLD by directly inhibiting the hyperactivation of immune cells.
In view of the crucial role of FASN in aggravating the disease conditions of NAFLD, several studies have conducted targeted FASN therapy for NAFLD and found that the pharmacological inhibition of FASN activity can significantly alleviate NAFLD. For example, some natural plant medicines, such as Schisandrin B, Arteether, and Limonin, can effectively inhibit the activation or infiltration of M1 macrophages, as well as the secretion of proinflammatory factors, by inhibiting FASN-mediated new fat formation and the lipolysis process, thereby exerting a protective effect on NAFLD [105,106,107,108,109]. As is known, M1 macrophages play a critical role in the pathogenesis of NAFLD by expressing high levels of pro-inflammatory cytokines and producing large amounts of reactive oxygen species and nitrogen substances [110]. These actions promote steatosis, inflammation, and hepatocyte damage. Moreover, the activation of macrophages can recruit the accumulation of other nonresident inflammatory cells, including B lymphocytes, T lymphocytes, and neutrophils, which jointly participate in liver inflammation and promote the progression of NAFLD [111]. Thus, FASN inhibition is emerging as an attractive therapeutic potential in the treatment of NAFLD due to targeting macrophages (Fig. 4).
Atherosclerosis
Atherosclerosis (AS) is a chronic inflammatory disease caused by abnormal accumulation of lipids. Eukaryotic initiation factor 6 (Eif6), a rate-limiting factor for protein translation, can widely affect cell metabolism; deletion of Eif6 can reduce AS by inhibiting FASN and suppressing lipid metabolism in macrophages [59]. In addition, miR-15a-5p can directly target the 3’ UTR of FASN mRNA and restrain the expression of FASN, thereby alleviating the inflammatory response and arterial injury in diabetic AS rats [112]. These studies showed that FASN participates in the pathogenesis of AS (Fig. 4).
Outstanding questions
Although existing studies have shown that FASN and FASN-mediated fatty acid metabolism participate in regulating the development of a variety of diseases, there are still many gaps in the mechanism of FASN action in immune-related diseases that need to be studied. Available data indicate that targeting and inhibiting FASN activity holds great promise in treating immune-associated diseases, but a great deal of work remains to be done to clarify the uncertainties that may arise from targeted inhibition of FASN. At the same time, further mechanistic studies are needed to determine the specific function of FASN and FASN-mediated fatty acid synthesis in regulating the immune microenvironment to reveal more therapeutic targets and deepen our understanding of the nosogenesis of immune-related diseases.
Conclusion and perspective
Disrupted fatty acid metabolism is closely related to the pathogenesis of numerous diseases. FASN exerts regulatory control over immune cell survival, activation, differentiation, and function. Consequently, it contributes to the onset and progression of various conditions, including tumors, cardiovascular diseases, inflammatory diseases, autoimmune diseases, infectious diseases, and other pathological states. Moreover, FASN is becoming a potential target for various diseases. Therefore, on the one hand, we need to further clarify the specific mechanisms by which FASN promotes or alleviates the pathogenesis of immune-related diseases by regulating the function of immune cells. On the other hand, targeting FASN to treat immune-related diseases still requires more clinical data. Therefore, developing safer, more economical, and more efficient drugs has become a research hotspot.
References
Gonzalez-Bohorquez D, Gallego Lopez IM, Jaeger BN, Pfammatter S, Bowers M, Semenkovich CF, et al. FASN-dependent de novo lipogenesis is required for brain development. Proc Natl Acad Sci USA. 2022;119:e2112040119.
Fhu CW, Ali A. Fatty acid synthase: an emerging target in cancer. Molecules. 2020;25:3935.
Vanauberg D, Schulz C, Lefebvre T. Involvement of the pro-oncogenic enzyme fatty acid synthase in the hallmarks of cancer: a promising target in anti-cancer therapies. Oncogenesis. 2023;12:16.
Ferraro GB, Ali A, Luengo A, Kodack DP, Deik A, Abbott KL, et al. Fatty acid synthesis is required for breast cancer brain metastasis. Nat Cancer. 2021;2:414–28.
Lim SA, Wei J, Nguyen TM, Shi H, Su W, Palacios G, et al. Lipid signalling enforces functional specialization of T(reg) cells in tumours. Nature. 2021;591:306–11.
Cao K, Zhang T, Li Z, Song M, Li A, Yan J, et al. Glycolysis and de novo fatty acid synthesis cooperatively regulate pathological vascular smooth muscle cell phenotypic switching and neointimal hyperplasia. J Pathol. 2023;259:388–401.
Wei H, Zhen L, Wang S, Zhang Y, Wang K, Jia P, et al. De novo lipogenesis in astrocytes promotes the repair of blood-brain barrier after transient cerebral ischemia through interleukin-33. Neuroscience. 2022;481:85–98.
Kim YC, Lee SE, Kim SK, Jang HD, Hwang I, Jin S, et al. Toll-like receptor mediated inflammation requires FASN-dependent MYD88 palmitoylation. Nat Chem Biol. 2019;15:907–16.
Xiong W, Sun KY, Zhu Y, Zhang X, Zhou YH, Zou X. Metformin alleviates inflammation through suppressing FASN-dependent palmitoylation of Akt. Cell Death Dis. 2021;12:934.
Aliyari SR, Ghaffari AA, Pernet O, Parvatiyar K, Wang Y, Gerami H, et al. Suppressing fatty acid synthase by type I interferon and chemical inhibitors as a broad spectrum anti-viral strategy against SARS-CoV-2. Acta Pharm Sin B. 2022;12:1624–35.
Williams CG, Jureka AS, Silvas JA, Nicolini AM, Chvatal SA, Carlson-Stevermer J, et al. Inhibitors of VPS34 and fatty-acid metabolism suppress SARS-CoV-2 replication. Cell Rep. 2021;36:109479.
Young KE, Flaherty S, Woodman KM, Sharma-Walia N, Reynolds JM. Fatty acid synthase regulates the pathogenicity of Th17 cells. J Leukoc Biol. 2017;102:1229–35.
Berger AH, Bratland E, Sjogren T, Heimli M, Tyssedal T, Bruserud O, et al. Transcriptional changes in regulatory T cells from patients with autoimmune polyendocrine syndrome type 1 suggest functional impairment of lipid metabolism and gut homing. front immunol. 2021;12:722860.
Qian X, Yang Z, Mao E, Chen E. Regulation of fatty acid synthesis in immune cells. Scand J Immunol. 2018;88:e12713.
Cheng C, Geng F, Li Z, Zhong Y, Wang H, Cheng X, et al. Ammonia stimulates SCAP/Insig dissociation and SREBP-1 activation to promote lipogenesis and tumour growth. Nat Metab. 2022;4:575–88.
Deng J, Peng M, Zhou S, Xiao D, Hu X, Xu S, et al. Metformin targets Clusterin to control lipogenesis and inhibit the growth of bladder cancer cells through SREBP-1c/FASN axis. Signal Transduct Target Ther. 2021;6:98.
Menendez JA, Lupu R. Fatty acid synthase: a druggable driver of breast cancer brain metastasis. Expert Opin Ther Targets. 2022;26:427–44.
Tibbo AJ, Hartley A, Vasan R, Shaw R, Galbraith L, Mui E, et al. MBTPS2 acts as a regulator of lipogenesis and cholesterol synthesis through SREBP signalling in prostate cancer. Br J Cancer. 2023;128:1991–9.
Zhao M, Bu Y, Feng J, Zhang H, Chen Y, Yang G, et al. SPIN1 triggers abnormal lipid metabolism and enhances tumor growth in liver cancer. Cancer Lett. 2020;470:54–63.
Zeng H, Qin H, Liao M, Zheng E, Luo X, Xiao A, et al. CD36 promotes de novo lipogenesis in hepatocytes through INSIG2-dependent SREBP1 processing. Mol Metab. 2022;57:101428.
Mesquita I, Ferreira C, Moreira D, Kluck GEG, Barbosa AM, Torrado E, et al. The absence of HIF-1alpha increases susceptibility to leishmania donovani infection via activation of BNIP3/mTOR/SREBP-1c axis. Cell Rep. 2020;30:4052–64.e7.
Meng Z, Liu Q, Sun F, Qiao L. Hepatitis C virus nonstructural protein 5A perturbs lipid metabolism by modulating AMPK/SREBP-1c signaling. Lipids Health Dis. 2019;18:191.
Wang Y, Zhang Y, Wang Z, Yu L, Chen K, Xie Y, et al. The interplay of transcriptional coregulator NUPR1 with SREBP1 promotes hepatocellular carcinoma progression via upregulation of lipogenesis. Cell Death Discov. 2022;8:431.
Zhang Y, Sun Y, Zhang Y, Miao Q, Wang Q, Yang B, et al. Nuclear factor Y participates in alcoholic liver disease by activating SREBP1 expression in mice. Biochem Biophys Res Commun. 2021;541:90–4.
Li X, Chen YT, Hu P, Huang WC. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Mol Cancer Ther. 2014;13:855–66.
Lu Y, Zhang C, Song Y, Chen L, Chen X, Zheng G, et al. Gallic acid impairs fructose-driven de novo lipogenesis and ameliorates hepatic steatosis via AMPK-dependent suppression of SREBP-1/ACC/FASN cascade. Eur J Pharm. 2023;940:175457.
Shirouchi B, Yanagi S, Okawa C, Koga M, Sato M. 6-Ketocholestanol suppresses lipid accumulation by decreasing FASN gene expression through SREBP-dependent regulation in HepG2 cells. Cytotechnology. 2020;72:175–87.
Kim Y, Jee W, An EJ, Ko HM, Jung JH, Na YC, et al. Timosaponin A3 inhibits palmitate and stearate through suppression of SREBP-1 in pancreatic cancer. Pharmaceutics. 2022;14:945.
Zhang Q, Zhou X, Zhang J, Li Q, Qian Z. Selenium and vitamin B(6) cosupplementation improves dyslipidemia and fatty liver syndrome by SIRT1/SREBP-1c pathway in hyperlipidemic Sprague-Dawley rats induced by high-fat diet. Nutr Res. 2022;106:101–18.
Yu L, Wei J, Liu P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin Cancer Biol. 2022;85:69–94.
Vaidyanathan S, Salmi TM, Sathiqu RM, McConville MJ, Cox AG, Brown KK. YAP regulates an SGK1/mTORC1/SREBP-dependent lipogenic program to support proliferation and tissue growth. Dev Cell. 2022;57:719–31.e8.
Hu M, Chen Y, Deng F, Chang B, Luo J, Dong L, et al. D-Mannose regulates hepatocyte lipid metabolism via PI3K/Akt/mTOR signaling pathway and ameliorates hepatic steatosis in alcoholic liver disease. Front Immunol. 2022;13:877650.
Zhu L, Du W, Liu Y, Cheng M, Wang X, Zhang C, et al. Prolonged high-glucose exposure decreased SREBP-1/FASN/ACC in schwann cells of diabetic mice via blocking PI3K/Akt pathway. J Cell Biochem. 2019;120:5777–89.
Liao X, Song L, Zhang L, Wang H, Tong Q, Xu J, et al. LAMP3 regulates hepatic lipid metabolism through activating PI3K/Akt pathway. Mol Cell Endocrinol. 2018;470:160–7.
Zhao J, Zhang X, Gao T, Wang S, Hou Y, Yuan P, et al. SIK2 enhances synthesis of fatty acid and cholesterol in ovarian cancer cells and tumor growth through PI3K/Akt signaling pathway. Cell Death Dis. 2020;11:25.
Yu W, Ling J, Yu H, Du J, Liu T. AZGP1 suppresses the process of colorectal cancer after upregulating FASN expression via mTOR signal pathway. Gen Physiol Biophys. 2020;39:239–48.
McClellan B, Gries P, Harlow B, Tiziani S, Jolly C, deGraffenried L. An IGF-1R-mTORC1-SRPK2 signaling axis contributes to FASN regulation in breast cancer. BMC Cancer. 2022;22:976.
Xie P, Peng Z, Chen Y, Li H, Du M, Tan Y, et al. Neddylation of PTEN regulates its nuclear import and promotes tumor development. Cell Res. 2021;31:291–311.
Wang N, Chen S, Zhang B, Li S, Jin F, Gao D, et al. 8u, a pro-apoptosis/cell cycle arrest compound, suppresses invasion and metastasis through HSP90alpha downregulating and PI3K/Akt inactivation in hepatocellular carcinoma cells. Sci Rep. 2018;8:309.
El-Saudi AM, Altouhamy MA, Shaaban S, Badria FA, Youssef MM, El-Senduny FF. Down regulation of fatty acid synthase via inhibition of PI3K/AKT/mTOR in ovarian cancer cell line by novel organoselenium pseudopeptide. Curr Res Pharm Drug Discov. 2022;3:100134.
Sun Y, Guo W, Guo Y, Lin Z, Wang D, Guo Q, et al. Apoptosis induction in human prostate cancer cells related to the fatty acid metabolism by wogonin-mediated regulation of the AKT-SREBP1-FASN signaling network. Food Chem Toxicol. 2022;169:113450.
Zhang C, Sheng L, Yuan M, Hu J, Meng Y, Wu Y, et al. Orlistat delays hepatocarcinogenesis in mice with hepatic co-activation of AKT and c-Met. Toxicol Appl Pharm. 2020;392:114918.
Saw PE, Xu X, Chen J, Song EW. Non-coding RNAs: the new central dogma of cancer biology. Sci China Life Sci. 2021;64:22–50.
Slack FJ, Chinnaiyan AM. The role of non-coding RNAs in oncology. Cell. 2019;179:1033–55.
Zhang H, Wang R, Tang X, Li J, Li J, Wang M. FASN Targeted by miR-497-5p Regulates Cell Behaviors in Cervical Cancer. Nutr Cancer. 2022;74:3026–34.
Li X, He S, Wu P, Zhou Y, Long K, Wang T. MiR-328-5p inhibits the adipogenic differentiation of hMSCs by targeting fatty acid synthase. Folia Histochem Cytobiol. 2022;60:292–300.
Zhang M, Tang Y, Tang E, Lu W. MicroRNA-103 represses hepatic de novo lipogenesis and alleviates NAFLD via targeting FASN and SCD1. Biochem Biophys Res Commun. 2020;524:716–22.
Li H, Chen Z, Zhang Y, Yuan P, Liu J, Ding L, et al. MiR-4310 regulates hepatocellular carcinoma growth and metastasis through lipid synthesis. Cancer Lett. 2021;519:161–71.
Wang X, Yung MMH, Sharma R, Chen F, Poon YT, Lam WY, et al. Epigenetic silencing of miR-33b promotes peritoneal metastases of ovarian cancer by modulating the TAK1/FASN/CPT1A/NF-kappaB axis. Cancers. 2021;13:4795.
Lu X, Xia H, Jiang J, Xu X, Li M, Chen Z, et al. MicroRNA-212 targets SIRT2 to influence lipogenesis in bovine mammary epithelial cell line. J Dairy Res. 2020;87:232–8.
He Y, Xu S, Qi Y, Tian J, Xu F. Long noncoding RNA SNHG25 promotes the malignancy of endometrial cancer by sponging microRNA-497-5p and increasing FASN expression. J Ovarian Res. 2021;14:163.
Chen Q, Yang Z, Ding H, Li H, Wang W, Pan Z. CircWHSC1 promotes breast cancer progression by regulating the FASN/AMPK/mTOR axis through sponging miR-195-5p. Front Oncol. 2021;11:649242.
Yu X, Tong H, Chen J, Tang C, Wang S, Si Y, et al. CircRNA MBOAT2 promotes intrahepatic cholangiocarcinoma progression and lipid metabolism reprogramming by stabilizing PTBP1 to facilitate FASN mRNA cytoplasmic export. Cell Death Dis. 2023;14:20.
Song Y, Wang J, Xu J, Gao Y, Xu Z. Circ_0018909 knockdown inhibits the development of pancreatic cancer via the miR-545-3p/FASN axis and reduces macrophage polarization to M2. J Biochem Mol Toxicol. 2023;37:e23293.
Li L, Zhang X, Ren H, Huang X, Shen T, Tang W, et al. miR-23a/b-3p promotes hepatic lipid accumulation by regulating Srebp-1c and Fas. J Mol Endocrinol. 2021;68:35–49.
Kanagasabai T, Li G, Shen TH, Gladoun N, Castillo-Martin M, Celada SI, et al. MicroRNA-21 deficiency suppresses prostate cancer progression through downregulation of the IRS1-SREBP-1 signaling pathway. Cancer Lett. 2022;525:46–54.
Wang H, Chen Y, Liu Y, Li Q, Luo J, Wang L, et al. The lncRNA ZFAS1 regulates lipogenesis in colorectal cancer by binding polyadenylate-binding protein 2 to stabilize SREBP1 mRNA. Mol Ther Nucleic Acids. 2022;27:363–74.
Li Q, Yao H, Wang Y, Wu Y, Thorne RF, Zhu Y, et al. circPRKAA1 activates a Ku80/Ku70/SREBP-1 axis driving de novo fatty acid synthesis in cancer cells. Cell Rep. 2022;41:111707.
Chen Y, Wang Z, Xian X, Zhuang Y, Chang J, Zhan X, et al. Eukaryotic initiation factor 6 repression mitigates atherosclerosis progression by inhibiting macrophages expressing Fasn. IUBMB Life. 2023;75:440–52.
Zheng Z, Shang Y, Xu R, Yan X, Wang X, Cai J, et al. Ubiquitin specific peptidase 38 promotes the progression of gastric cancer through upregulation of fatty acid synthase. Am J Cancer Res. 2022;12:2686–96.
Balamurugan K, Medishetti R, Kotha J, Behera P, Chandra K, Mavuduru VA, et al. PHLPP1 promotes neutral lipid accumulation through AMPK/ChREBP-dependent lipid uptake and fatty acid synthesis pathways. iScience. 2022;25:103766.
Gu L, Zhu Y, Lin X, Tan X, Lu B, Li Y. Stabilization of FASN by ACAT1-mediated GNPAT acetylation promotes lipid metabolism and hepatocarcinogenesis. Oncogene. 2020;39:2437–49.
Chen S, Saeed A, Liu Q, Jiang Q, Xu H, Xiao GG, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8:207.
Mosser DM, Hamidzadeh K, Goncalves R. Macrophages and the maintenance of homeostasis. Cell Mol Immunol. 2021;18:579–87.
Carroll RG, Zasłona Z, Galván-Peña S, Koppe EL, Sévin DC, Angiari S, et al. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J Biol Chem. 2018;293:5509–21.
Wei X, Song H, Yin L, Rizzo MG, Sidhu R, Covey DF, et al. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016;539:294–8.
Moon JS, Lee S, Park MA, Siempos II, Haslip M, Lee PJ, et al. UCP2-induced fatty acid synthase promotes NLRP3 inflammasome activation during sepsis. J Clin Invest. 2015;125:665–80.
Kumar A, Gupta P, Rana M, Chandra T, Dikshit M, Barthwal MK. Role of pyruvate kinase M2 in oxidized LDL-induced macrophage foam cell formation and inflammation. J Lipid Res. 2020;61:351–64.
Wang HW, Kok SH, Yang CN, Hong CY, Chi CW, Chen MH, et al. Blockade of fatty acid signalling inhibits lipopolysaccharide-induced macrophage recruitment and progression of apical periodontitis. Int Endod J. 2021;54:902–15.
Zhou Y, Su W, Liu H, Chen T, Hoti N, Pei H, et al. Fatty acid synthase is a prognostic marker and associated with immune infiltrating in gastric cancers precision medicine. Biomark Med. 2020;14:185–99.
Philip M, Schietinger A. CD8(+) T cell differentiation and dysfunction in cancer. Nat Rev Immunol. 2022;22:209–23.
Krovi SH, Kuchroo VK. Activation pathways that drive CD4(+) T cells to break tolerance in autoimmune diseases. Immunol Rev. 2022;307:161–90.
Almeida L, Dhillon-LaBrooy A, Carriche G, Berod L, Sparwasser T. CD4(+) T-cell differentiation and function: Unifying glycolysis, fatty acid oxidation, polyamines NAD mitochondria. J Allergy Clin Immunol. 2021;148:16–32.
Du L, Ho BM, Zhou L, Yip YWY, He JN, Wei Y, et al. Growth hormone releasing hormone signaling promotes Th17 cell differentiation and autoimmune inflammation. Nat Commun. 2023;14:3298.
Voss K, Luthers CR, Pohida K, Snow AL. Fatty acid synthase contributes to restimulation-induced cell death of human CD4 T cells. Front Mol Biosci. 2019;6:106.
Wang K, Fu W. Transcriptional regulation of Treg homeostasis and functional specification. Cell Mol Life Sci. 2020;77:4269–87.
Sun P, Zhang X, Wang RJ, Ma QY, Xu L, Wang Y, et al. PI3Kalpha inhibitor CYH33 triggers antitumor immunity in murine breast cancer by activating CD8(+)T cells and promoting fatty acid metabolism. J Immunother Cancer. 2021;9:e003093.
Del Prete A, Salvi V, Soriani A, Laffranchi M, Sozio F, Bosisio D, et al. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol Immunol. 2023;20:432–47.
Marvin J, Rhoads JP, Major AS. FcgammaRIIb on CD11c(+) cells modulates serum cholesterol and triglyceride levels and differentially affects atherosclerosis in male and female Ldlr(-/-) mice. Atherosclerosis. 2019;285:108–19.
Jiang L, Fang X, Wang H, Li D, Wang X. Ovarian cancer-intrinsic fatty acid synthase prevents anti-tumor immunity by disrupting tumor-infiltrating dendritic cells. Front Immunol. 2018;9:2927.
Vinuesa CG, Grenov A, Kassiotis G. Innate virus-sensing pathways in B cell systemic autoimmunity. Science. 2023;380:478–84.
Luo W, Adamska JZ, Li C, Verma R, Liu Q, Hagan T, et al. SREBP signaling is essential for effective B cell responses. Nat Immunol. 2023;24:337–48.
Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–66.
Zhang M, Yu L, Sun Y, Hao L, Bai J, Yuan X, et al. Comprehensive analysis of FASN in tumor immune infiltration and prognostic value for immunotherapy and promoter DNA methylation. Int J Mol Sci. 2022;23:15603.
Wang Q, Tian N, Zhang W, Lin Z, Shi F, Kong Y, et al. Fatty acid synthase mutations predict favorable immune checkpoint inhibitor outcome and response in melanoma and non-small cell lung cancer patients. Cancers. 2022;14:5638.
Bizymi N, Matthaiou AM, Matheakakis A, Voulgari I, Aresti N, Zavitsanou K, et al. New perspectives on myeloid-derived suppressor cells and their emerging role in haematology. J Clin Med. 2022;11:5326.
Zhang H, Chen H, Yin S, Fan L, Jin C, Zhao C, et al. Docosahexaenoic acid reverses PD-L1-mediated immune suppression by accelerating its ubiquitin-proteasome degradation. J Nutr Biochem. 2023;112:109186.
Shen M, Tsai Y, Zhu R, Keng PC, Chen Y, Chen Y, et al. FASN-TGF-beta1-PD-L1 axis contributes to the development of resistance to NK cell cytotoxicity of cisplatin-resistant lung cancer cells. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:313–22.
Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression- implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16:356–71.
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40.
Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133–46.
Gao Y, Hu JH, Liang XD, Chen J, Liu CC, Liu YY, et al. Curcumin inhibits classical swine fever virus replication by interfering with lipid metabolism. Vet Microbiol. 2021;259:109152.
Aweya JJ, Zheng X, Zheng Z, Wang W, Fan J, Yao D, et al. The sterol regulatory element binding protein homolog of Penaeus vannamei modulates fatty acid metabolism and immune response. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158757.
Shirasaki T, Honda M, Shimakami T, Horii R, Yamashita T, Sakai Y, et al. MicroRNA-27a regulates lipid metabolism and inhibits hepatitis C virus replication in human hepatoma cells. J Virol. 2013;87:5270–86.
Zheng Q, Huang Y, Wang L, Zhang Y, Guo X, Huang X, et al. SGIV induced and exploited cellular de novo fatty acid synthesis for virus entry and replication. Viruses. 2022;14:180.
Chu J, Xing C, Du Y, Duan T, Liu S, Zhang P, et al. Pharmacological inhibition of fatty acid synthesis blocks SARS-CoV-2 replication. Nat Metab. 2021;3:1466–75.
Cao F, Liu YC, Ni QY, Chen Y, Wan CH, Liu SY, et al. Temporal trends in the prevalence of autoimmune diseases from 1990 to 2019. Autoimmun Rev. 2023;22:103359.
He C, Wang Y, Wen Y, Li T, Hu E, Zeng S, et al. Quantitative proteomic analysis of Bi Zhong Xiao decoction against collagen-induced arthritis rats in the early and late stages. BMC Complement Med Ther. 2022;22:186.
Hegarty LM, Jones GR, Bain CC. Macrophages in intestinal homeostasis and inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2023;20:538–53.
Roca-Lema D, Quiroga M, Khare V, Diaz-Diaz A, Barreiro-Alonso A, Rodriguez-Alonso A, et al. Role of the E3 ubiquitin-ligase Hakai in intestinal inflammation and cancer bowel disease. Sci Rep. 2022;12:17571.
Matsuo S, Yang WL, Aziz M, Kameoka S, Wang P. Fatty acid synthase inhibitor C75 ameliorates experimental colitis. Mol Med. 2014;20:1–9.
Sun T, Liu Y, Liu L, Ma F. MicroRNA-544 attenuates diabetic renal injury via suppressing glomerulosclerosis and inflammation by targeting FASN. Gene. 2020;723:143986.
Wang K, Li L, Jin J, An Y, Wang Z, Zhou S, et al. Fatty acid synthase (Fasn) inhibits the expression levels of immune response genes via alteration of alternative splicing in islet cells. J Diabetes Complicat. 2022;36:108159.
O’Farrell M, Duke G, Crowley R, Buckley D, Martins EB, Bhattacharya D, et al. FASN inhibition targets multiple drivers of NASH by reducing steatosis, inflammation and fibrosis in preclinical models. Sci Rep. 2022;12:15661.
Ma R, Zhan Y, Zhang Y, Wu L, Wang X, Guo M. Schisandrin B ameliorates non-alcoholic liver disease through anti-inflammation activation in diabetic mice. Drug Dev Res. 2022;83:735–44.
Xu J, He X, Huang X, Zhang F, Ren X, Asakiya C, et al. Artemether ameliorates non-alcoholic steatohepatitis by repressing lipogenesis, inflammation, and fibrosis in mice. Front Pharm. 2022;13:851342.
Li Y, Yang M, Lin H, Yan W, Deng G, Ye H, et al. Limonin alleviates non-alcoholic fatty liver disease by reducing lipid accumulation, suppressing inflammation and oxidative stress. Front Pharm. 2021;12:801730.
Xu Z, Hu W, Wang B, Xu T, Wang J, Wei D. Canagliflozin ameliorates nonalcoholic fatty liver disease by regulating lipid metabolism and inhibiting inflammation through induction of autophagy. Yonsei Med J. 2022;63:619–31.
Oh S, Son M, Byun KA, Jang JT, Choi CH, Son KH, et al. Attenuating effects of dieckol on high-fat diet-induced nonalcoholic fatty liver disease by decreasing the NLRP3 inflammasome and pyroptosis. Mar Drugs. 2021;19:318.
Barreby E, Chen P, Aouadi M. Macrophage functional diversity in NAFLD - more than inflammation. Nat Rev Endocrinol. 2022;18:461–72.
Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–59.
Liu Y, Liu LY, Jia Y, Sun YY, Ma FZ. Role of microRNA-15a-5p in the atherosclerotic inflammatory response and arterial injury improvement of diabetic by targeting FASN. Biosci Rep. 2019;39:BSR20181852.
Funding
This work was supported by grants from Tai Shan Young Scholar Foundation of Shandong Province (tsqn202211234), the National Natural Science Foundation of China (NO. 82071824, 81901655), Shandong Provincial Youth Innovation Technology Support Program (2021KJ074), and Jining Medical University high-level scientific research project cultivation plan (JYGC2022KJ005).
Author information
Authors and Affiliations
Contributions
YCX, YHY, and GJD conceived of and designed the overall study; YCX and YHY collected information, and prepared figures; HBX and GJD performed a systematic literature search, and drafted manuscript; all authors revised the manuscript critically and approved final version of manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Professor Alessandro Finazzi-Agrò
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiao, Y., Yang, Y., Xiong, H. et al. The implications of FASN in immune cell biology and related diseases. Cell Death Dis 15, 88 (2024). https://doi.org/10.1038/s41419-024-06463-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-024-06463-6