Influence of the Mixtures of Vegetable Oil and Vitamin E over the Microstructure and Rheology of Organogels
Abstract
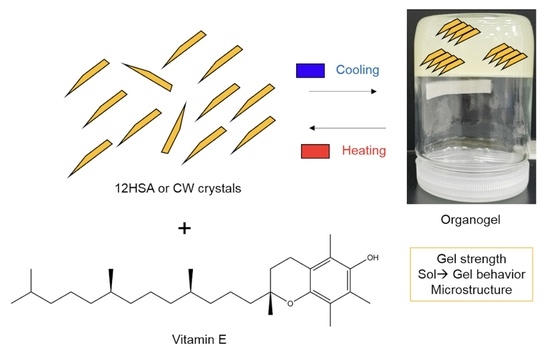
:1. Introduction
2. Results and Discussion
SAXS Model
3. Conclusions
4. Materials and Methods
4.1. Organogel Preparation
4.2. Microscopy Tests
4.3. Rheological Characterization
4.4. SAXS
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, promising drug delivery systems: An update of state-of-the-art and recent applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef]
- Rogers, M.A.; Marangoni, A.G. Acid in Vegetable Oils & DESIGN 2008. Cryst. Growth Des. 2008, 8, 4596–4601. [Google Scholar]
- Toro-Vazquez, J.F.; Morales-Rueda, J.; Torres-Martínez, A.; Charó-Alonso, M.A.; Mallia, V.A.; Weiss, R.G. Cooling rate effects on the microstructure, solid content, and rheological properties of organogels of amides derived from stearic and (R)-12- hydroxystearic acid in vegetable oil. Langmuir 2013, 29, 7642–7654. [Google Scholar] [CrossRef] [PubMed]
- Co, E.D.; Marangoni, A.G. Organogels: An alternative edible oil-structuring method. J. Am. Oil Chem. Soc. 2012, 89, 749–780. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.; Alonzo-Macias, M.; González-Chávez, M. Thermal and textural properties of organogels developed by candelilla wax in safflower oil. J. Am. Oil Chem. Soc. 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.A.; Weiss, R.G.; Toro-Vazquez, J.F. Thermo-mechanical properties of candelilla wax and dotriacontane organogels in safflower oil. Eur. J. Lipid Sci. Technol. 2009, 111, 207–215. [Google Scholar] [CrossRef]
- Rogers, M.A.; Wright, A.J.; Marangoni, A.G. Crystalline stability of self-assembled fibrillar networks of 12-hydroxystearic acid in edible oils. Food Res. Int. 2008, 41, 1026–1034. [Google Scholar] [CrossRef]
- Dalkas, G.; Matheson, A.B.; Vass, H.; Gromov, A.; Lloyd, G.O.; Koutsos, V.; Clegg, P.S.; Euston, S.R. Molecular Interactions behind the Self-Assembly and Microstructure of Mixed Sterol Organogels. Langmuir 2018, 34, 8629–8638. [Google Scholar] [CrossRef]
- Matheson, A.B.; Dalkas, G.; Gromov, A.; Euston, S.R.; Clegg, P.S. The development of phytosterol-lecithin mixed micelles and organogels. Food Funct. 2017, 8, 4547–4554. [Google Scholar] [CrossRef] [Green Version]
- Martinez, R.M.; Magalhães, W.V.; Sufi, B.S.; Padovani, G.; Nazato, L.I.S.; Velasco, M.V.R.; da Silva Lannes, S.C.; Baby, A.R. Vitamin E-loaded bigels and emulsions: Physicochemical characterization and potential biological application. Colloids Surf. B Biointerfaces 2021, 201, 111651. [Google Scholar] [CrossRef]
- Butt, H.; Mehmood, A.; Ali, M.; Tasneem, S.; Anjum, M.S.; Tarar, M.N.; Khan, S.N.; Riazuddin, S. Protective role of vitamin E preconditioning of human dermal fibroblasts against thermal stress in vitro. Life Sci. 2017, 184, 70. [Google Scholar] [CrossRef]
- Delinasios, G.J.; Karbaschi, M.; Cooke, M.S.; Young, A.R. Vitamin E inhibits the UVAI induction of “light” and “dark” cyclobutane pyrimidine dimers, and oxidatively generated DNA damage, in keratinocytes. Sci. Rep. 2018, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Lio, P.A. Alternative Treatments for Atopic Dermatitis: An Update. Am. J. Clin. Dermatol. 2019, 20, 251–266. [Google Scholar] [CrossRef]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Oxidative stability of flaxseed oil: Effect of hydrophilic, hydrophobic and intermediate polarity antioxidants. Food Chem. 2018, 266, 524–533. [Google Scholar] [CrossRef]
- Puscas, A.; Muresan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A Review of Current and Potential Applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsaab, H.; Bonam, S.P.; Bahl, D.; Chowdhury, P.; Alexander, K.; Boddu, S.H.S. Organogels in drug delivery: A special emphasis on organogels pluronic lecithin. J. Pharm. Pharm. Sci. 2016, 19, 252–273. [Google Scholar] [CrossRef]
- Rocha, J.C.B.; Lopes, J.D.; Mascarenhas, M.C.N.; Arellano, D.B.; Guerreiro, L.M.R.; da Cunha, R.L. Thermal and rheological properties of organogels formed by sugarcane or candelilla wax in soybean oil. Food Res. Int. 2013, 50, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.R.; Babaahmadi, M.; Lesaffer, A.; Dewettinck, K. Rheological Profiling of Organogels Prepared at Critical Gelling Concentrations of Natural Waxes in a Triacylglycerol Solvent. J. Agric. Food Chem. 2015, 63, 4862–4869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupi, F.R.; Gentile, L.; Gabriele, D.; Mazzulla, S.; Baldino, N.; de Cindio, B. Olive oil and hyperthermal water bigels for cosmetic uses. J. Colloid Interface Sci. 2015, 459, 70–78. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Pang, Z. Tribo-rheological properties of acid milk gels with different types of gelatin: Effect of concentration. J. Dairy Sci. 2019, 102, 7849–7862. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Nishinari, K. “Weak gel”-type rheological properties of aqueous dispersions of nonaggregated κ-carrageenan helices. J. Agric. Food Chem. 2001, 49, 4436–4441. [Google Scholar] [CrossRef] [PubMed]
- Avanza, M.V.; Puppo, M.C.; Añón, M.C. Rheological characterization of amaranth protein gels. Food Hydrocoll. 2005, 19, 889–898. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Scholten, E. Self-assemblies of lecithin and α-tocopherol as gelators of lipid material. RSC Adv. 2014, 4, 2466–2473. [Google Scholar] [CrossRef] [Green Version]
- Zare, Y.; Park, S.P.; Rhee, K.Y. Analysis of complex viscosity and shear thinning behavior in poly (lactic acid)/poly (ethylene oxide)/carbon nanotubes biosensor based on Carreau–Yasuda model. Results Phys. 2019, 13, 102245. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kulichikhin, V.G.; Malkin, A.Y. The rheological characterisation of typical injection implants based on hyaluronic acid for contour correction. Rheol. Acta 2016, 55, 223–233. [Google Scholar] [CrossRef]
- Carriço, C.; Pinto, P.; Graça, A.; Gonçalves, L.M.; Ribeiro, H.M.; Marto, J. Design and characterization of a new quercus suber-based pickering emulsion for topical application. Pharmaceutics 2019, 11, 131. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Liu, J.; Moussa, Z.L.; Collins, J.E.; McDonnell, S.; Hayward, A.M.; Jajoo, K.; Langer, R.; Traverso, G. Endoscopically Injectable Shear-Thinning Hydrogels Facilitating Polyp Removal. Adv. Sci. 2019, 6, 1901041. [Google Scholar] [CrossRef]
- Espinosa, L.; To, N.; Symoneaux, R.; Renard, C.M.G.C.; Biau, N.; Cuvelier, G. Effect of processing on rheological, structural and sensory properties of apple puree. Procedia Food Sci. 2011, 1, 513–520. [Google Scholar] [CrossRef]
- Valoppi, F.; Salmi, A.; Ratilainen, M.; Barba, L.; Puranen, T.; Tommiska, O.; Helander, P.; Heikkilä, J.; Haeggström, E. Controlling oleogel crystallization using ultrasonic standing waves. Sci. Rep. 2020, 10, 14448. [Google Scholar] [CrossRef]
- Lupi, F.R.; Shakeel, A.; Greco, V.; Oliviero Rossi, C.; Baldino, N.; Gabriele, D. A rheological and microstructural characterisation of bigels for cosmetic and pharmaceutical uses. Mater. Sci. Eng. C 2016, 69, 358–365. [Google Scholar] [CrossRef]
- Malvern Panalytical Multiple Ways to Optimize Rheology for Increased Dispersion, Colloidal and Emulsion Stability. Available online: https://www.azom.com/article.aspx?ArticleID=11442 (accessed on 10 August 2021).
- Carrà, S.; Chiozza, F.; Curto, F.; Ferraioli, M. Rheology to understand operational parameter, as a predictive method to avoid off specifications and maximizing the packaging process. Annu. Trans. Nord. Rheol. Soc. 2015, 23, 135–142. [Google Scholar]
- Okuro, P.K.; Martins, A.J.; Vicente, A.A.; Cunha, R.L. Perspective on oleogelator mixtures, structure design and behaviour towards digestibility of oleogels. Curr. Opin. Food Sci. 2020, 35, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Alghooneh, A.; Razavi, S.M.A.; Kasapis, S. A New Approach to Distinguish Thixotropic and Viscoelastic Phenomena. Food Biophys. 2020, 15, 72–84. [Google Scholar] [CrossRef]
- Estanqueiro, M.; Amaral, M.H.; Sousa Lobo, J.M. Comparison between sensory and instrumental characterization of topical formulations: Impact of thickening agents. Int. J. Cosmet. Sci. 2016, 38, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Toker, O.S.; Karasu, S.; Yilmaz, M.T.; Karaman, S. Three interval thixotropy test (3ITT) in food applications: A novel technique to determine structural regeneration of mayonnaise under different shear conditions. Food Res. Int. 2015, 70, 125–133. [Google Scholar] [CrossRef]
- Martinez, R.M.; Rosado, C.; Velasco, M.V.R.; Lannes, S.C.S.; Baby, A.R. Main features and applications of organogels in cosmetics. Int. J. Cosmet. Sci. 2019, 41, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Esposito, C.L.; Tardif, V.; Sarrazin, M.; Kirilov, P.; Roullin, V.G. Preparation and characterization of 12-HSA-based organogels as injectable implants for the controlled delivery of hydrophilic and lipophilic therapeutic agents. Mater. Sci. Eng. C 2020, 114, 110999. [Google Scholar] [CrossRef] [PubMed]
- Relkin, P.; Yung, J.M.; Kalnin, D.; Ollivon, M. Structural behaviour of lipid droplets in protein-stabilized nano-emulsions and stability of α-tocopherol. Food Biophys. 2008, 3, 163–168. [Google Scholar] [CrossRef]
- Kamal, M.A.; Raghunathan, V.A. Modulated phases of phospholipid bilayers induced by tocopherols. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2486–2493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.; Zhang, S.; Ma, H.; Dong, P.; Ma, X.; Xu, M.; Tian, Y.; Tang, Z.; Peng, J.; Chen, H.; et al. In situ monitoring of the structural change of microemulsions in simulated gastrointestinal conditions by SAXS and FRET. Acta Pharm. Sin. B 2018, 8, 655–665. [Google Scholar] [CrossRef]
- Takeno, H.; Mochizuki, T.; Yoshiba, K.; Kondo, S.; Dobashi, T. Self-assembling Structures and Sol-Gel Transition of Optically Active and Racemic 12-Hydroxystearic Acids in Organic Solvents. In Gels: Structures, Properties, and Functions. Progress in Colloid and Polymer Science; Tokita, M.N.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; p. 47. [Google Scholar]
- Blake, A.I.; Co, E.D.; Marangoni, A.G. Structure and physical properties of plant wax crystal networks and their relationship to oil binding capacity. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 885–903. [Google Scholar] [CrossRef]
- Chopin-Doroteo, M.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.A.; de la Peña-Gil, A.; Toro-Vazquez, J.F. The Effect of Shearing in the Thermo-mechanical Properties of Candelilla Wax and Candelilla Wax-Tripalmitin Organogels. Food Biophys. 2011, 6, 359–376. [Google Scholar] [CrossRef]
- Takeno, H.; Maehara, A.; Kuchiishi, M.; Yoshiba, K.; Takeshita, H.; Kondo, S.; Dobashi, T.; Takenaka, M.; Hasegawa, H. Structural and thermal properties of unpurified and purified 12-hydroxystearic acid solutions. J. Fiber Sci. Technol. 2012, 68, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, J.S. Analysis of small-angle scattering data from colloids and polymer solutions: Modeling and least-squares fitting. Adv. Colloid Interface Sci. 1997, 70, 171–210. [Google Scholar] [CrossRef]
- Sundblom, A.; Oliveira, C.L.P.; Palmqvist, A.E.C.; Pedersen, J.S. Modeling in situ small-angle X-ray scattering measurements following the formation of mesostructured silica. J. Phys. Chem. C 2009, 113, 7706–7713. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Hansen, S.; Bauer, R. The aggregation behavior of zinc-free insulin studied by small-angle neutron-scattering. Eur. Biophys. J. 1994, 22, 379–389. [Google Scholar] [CrossRef]
- Pinsky, M.A.; Karlin, S. An Introduction to Stochastic Modeling; Pinsky, M.A., Karlin, S., Eds.; Academic Press: Boston, MA, USA, 2011. [Google Scholar]
- Kissell, R.; Poserina, J. Optimal Sports Math, Statistics, and Fantasy; Kissell, R., Poserina, J., Eds.; Academic Press: Boston, MA, USA, 2017. [Google Scholar]
- De, S.; Krishnan, P.; van der Schaaf, J.; Kuipers, J.A.M.; Peters, E.A.J.F.; Padding, J.T. Viscoelastic effects on residual oil distribution in flows through pillared microchannels. J. Colloid Interface Sci. 2018, 510, 262–271. [Google Scholar] [CrossRef]
- Oliveira, C.L.P.; Behrens, M.A.; Pedersen, J.S.; Erlacher, K.; Otzen, D.; Pedersen, J.S. A SAXS Study of Glucagon Fibrillation. J. Mol. Biol. 2009, 387, 147–161. [Google Scholar] [CrossRef]







| Code | (Pa·s) | (Pa·s) | (s) | n | a | Tmelt (°C) | Tgel (°C) |
|---|---|---|---|---|---|---|---|
| CW2:VO98 | 9.37 ± 0.03 | 0.15 ± 0.01 | 0.39 ± 0.01 | −0.26 ± 0.01 | 2.15 ± 0.03 | 55 | 34 |
| CW2:VO93:VE5 | 16.06 ± 1.26 | 0.14 ± 0.01 | 0.38 ± 0.02 | −0.44 ± 0.33 | 1.24 ± 0.03 | 55 | 38 |
| CW2:VO78:VE20 | 24.93 ± 0.18 | 0.22 ± 0.01 | 0.41 ± 0.01 | −0.29 ± 0.02 | 2.49 ± 0.04 | 50 | 39 |
| 12HSA2:VO98 | 350.08 ± 8.80 | 2.19 ± 0.01 | 0.27 ± 0.01 | −1.65 ± 0.01 | 3.01 ± 0.01 | 72 | 66 |
| 12HSA2:VO93:VE5 | 284.41 ± 7.25 | 2.21 ± 0.01 | 0.29 ± 0.01 | −1.40 ± 0.01 | 3.63 ± 0.01 | 63 | 58 |
| 12HSA2:VO78:VE20 | 150.89 ± 4.09 | 2.94 ± 0.01 | 0.29 ± 0.01 | −1.39 ± 0.01 | 4.82 ± 0.01 | 59 | 46 |
| Code | % CW (w/w) | % 12HSA (w/w) | % VO (w/w) | % MO (w/w) | % VE (w/w) |
|---|---|---|---|---|---|
| CW2:VO98 | 2.0 | - | 98.0 | - | - |
| CW2:VO97:VE1 | 2.0 | - | 97.0 | - | 1.0 |
| CW2:VO96:VE2 | 2.0 | - | 96.0 | - | 2.0 |
| CW2:VO93:VE5 | 2.0 | - | 93.0 | - | 5.0 |
| CW2:VO78:VE20 | 2.0 | - | 78.0 | - | 20.0 |
| 12HSA2:VO98 | - | 2.0 | 98.0 | - | - |
| 12HSA2:VO97:VE1 | - | 2.0 | 97.0 | - | 1.0 |
| 12HSA2:VO96:VE2 | - | 2.0 | 96.0 | - | 2.0 |
| 12HSA2:VO93:VE5 | - | 2.0 | 93.0 | - | 5.0 |
| 12HSA2:VO78:VE20 | - | 2.0 | 78.0 | - | 20.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, R.M.; Oseliero Filho, P.L.; Gerbelli, B.B.; Magalhães, W.V.; Velasco, M.V.R.; da Silva Lannes, S.C.; de Oliveira, C.L.P.; Rosado, C.; Baby, A.R. Influence of the Mixtures of Vegetable Oil and Vitamin E over the Microstructure and Rheology of Organogels. Gels 2022, 8, 36. https://doi.org/10.3390/gels8010036
Martinez RM, Oseliero Filho PL, Gerbelli BB, Magalhães WV, Velasco MVR, da Silva Lannes SC, de Oliveira CLP, Rosado C, Baby AR. Influence of the Mixtures of Vegetable Oil and Vitamin E over the Microstructure and Rheology of Organogels. Gels. 2022; 8(1):36. https://doi.org/10.3390/gels8010036
Chicago/Turabian StyleMartinez, Renata Miliani, Pedro Leonidas Oseliero Filho, Barbara Bianca Gerbelli, Wagner Vidal Magalhães, Maria Valéria Robles Velasco, Suzana Caetano da Silva Lannes, Cristiano Luis Pinto de Oliveira, Catarina Rosado, and André Rolim Baby. 2022. "Influence of the Mixtures of Vegetable Oil and Vitamin E over the Microstructure and Rheology of Organogels" Gels 8, no. 1: 36. https://doi.org/10.3390/gels8010036