pH Modulated Formation of Complexes with Various Stoichiometry between Polymer Network and Fe(III) in Thermosensitive Gels Modified with Gallic Acid
Abstract
:1. Introduction
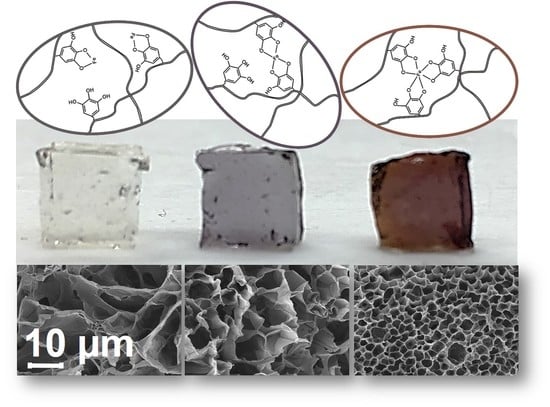
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. p(NIPA-X%APMA)-Gal Gel Preparation
4.3. Methods
4.3.1. Swelling Ratio Measurements
4.3.2. Scanning Electron Microscopy
4.3.3. UV-Vis Spectroscopy
4.3.4. Electrochemical Characterization
4.3.5. Rheological Measurements
4.3.6. NMR Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldberg, I.; Rokem, J.S. Organic and Fatty Acid Production, Microbial. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Acadaemic Press: Cambridge, MA, USA, 2009; pp. 421–442. [Google Scholar]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Saleh, S.M.M.; Mahmoud, A.B.; Al-Salahy, M.B.; Moustafa, F.A.M. Morphological, immunohistochemical, and biochemical study on the ameliorative effect of gallic acid against bisphenol A-induced nephrotoxicity in male albino rats. Sci. Rep. 2023, 13, 1732. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Díaz Hidalgo, R.J.; Córdoba, R.; Grigoryan, H.; Vieira, M.; Melo, M.J.; Nabais, P.; Otero, V.; Teixeira, N.; Fani, S.; Al-Abbady, H. The making of black inks in an Arabic treatise by al-Qalalūsī dated from the 13th c.: Reproduction and characterisation of iron-gall ink recipes. Herit. Sci. 2023, 11, 1–14. [Google Scholar] [CrossRef]
- Powell, H.K.J.; Taylor, M.C. Interactions of iron(II) and iron(III) with gallic acid and its homologues: A potentiometric and spectrophotometric study. Aust. J. Chem. 1982, 35, 739–756. [Google Scholar] [CrossRef]
- Fazary, A.I.; Taha, M.; Ju, Y.-H. Iron Complexation Studies of Gallic Acid. J. Chem. Eng. Data 2009, 54, 35–42. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Kim, S.-K.; Ahn, C.-B.; Je, J.-Y. Preparation, characterization, and antioxidant properties of gallic acid-grafted-chitosans. Carbohydr. Polym. 2011, 83, 1617–1622. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Sha, D.; Liu, Y.; Liu, Z.; Shi, K.; Liu, W.; Yu, C.; Ji, X. PVA/Poly(hexamethylene guanidine)/Gallic Acid Composite Hydrogel Films and Their Antibacterial Performance. ACS Appl. Polym. Mater. 2021, 3, 3867–3877. [Google Scholar] [CrossRef]
- Chuan, D.; Hou, H.; Wang, Y.; Mu, M.; Li, J.; Ren, Y.; Zhao, N.; Han, B.; Chen, H.; Guo, G. Multifunctional metal-polyphenol nanocomposite for melanoma targeted photo/chemodynamic synergistic therapy. J. Mater. Sci. Technol. 2023, 152, 159–168. [Google Scholar] [CrossRef]
- Kang, B.; Vales, T.P.; Cho, B.-K.; Kim, J.-K.; Kim, H.-J. Development of Gallic Acid-Modified Hydrogels Using Interpenetrating Chitosan Network and Evaluation of Their Antioxidant Activity. Molecules 2017, 22, 1976. [Google Scholar] [CrossRef]
- Phetcharat, P.; Sangsanoh, P.; Choipang, C.; Chaiarwut, S.; Suwantong, O.; Chuysinuan, P.; Supaphol, P. Curative Effects of Copper Iodide Embedded on Gallic Acid Incorporated in a Poly(vinyl alcohol) (PVA) Liquid Bandage. Gels 2023, 9, 53. [Google Scholar] [CrossRef]
- Gong, W.; Wang, R.; Huang, H.; Hou, Y.; Wang, X.; He, W.; Gong, X.; Hu, J. Construction of double network hydrogels using agarose and gallic acid with antibacterial and anti-inflammatory properties for wound healing. Int. J. Biol. Macromol. 2023, 227, 698–710. [Google Scholar] [CrossRef]
- He, Y.; Liu, K.; Guo, S.; Chang, R.; Zhang, C.; Guan, F.; Yao, M. Multifunctional hydrogel with reactive oxygen species scavenging and photothermal antibacterial activity accelerates infected diabetic wound healing. Acta Biomater. 2023, 155, 199–217. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, P.; Huang, C.; Zeng, R.; Yang, L.; Han, Z.; Qu, Y.; Zhang, C. Photothermal-promoted multi-functional dual network polysaccharide hydrogel adhesive for infected and susceptible wound healing. Carbohydr. Polym. 2021, 273, 118557. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Zheng, L.; Wu, J. A polyphenol-modified chitosan hybrid hydrogel with enhanced antimicrobial and antioxidant activities for rapid healing of diabetic wounds. Nano Res. 2023, 16, 905–916. [Google Scholar] [CrossRef]
- Park, G.R.; Gwak, M.A.; Choi, Y.H.; Park, W.H. pH-sensitive gallol-rich chitosan hydrogel beads for on-off controlled drug delivery. Int. J. Biol. Macromol. 2023, 240, 124346. [Google Scholar] [CrossRef]
- Yu, C.; Chen, X.; Zhu, W.; Li, L.; Peng, M.; Zhong, Y.; Naeem, A.; Zang, Z.; Guan, Y. Synthesis of Gallic Acid-Loaded Chitosan-Grafted-2-Acrylamido-2-Methylpropane Sulfonic Acid Hydrogels for Oral Controlled Drug Delivery: In Vitro Biodegradation, Antioxidant, and Antibacterial Effects. Gels 2022, 8, 806. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Jaishankar, A.; Harrington, M.J.; Fullenkamp, D.E.; Di Marco, G.; He, L.; McKinley, G.H.; Messersmith, P.B.; Lee, K.Y.C. Metal-coordination: Using one of nature’s tricks to control soft material mechanics. J. Mater. Chem. B 2014, 2, 2467–2472. [Google Scholar] [CrossRef]
- Shafiq, Z.; Cui, J.; Pastor-Pérez, L.; San Miguel, V.; Gropeanu, R.A.; Serrano, C.; del Campo, A. Bioinspired Underwater Bonding and Debonding on Demand. Angew. Chem. Int. Ed. 2012, 51, 4332–4335. [Google Scholar] [CrossRef]
- Harrington, M.J.; Masic, A.; Holten-Andersen, N.; Waite, J.H.; Fratzl, P. Iron-Clad Fibers: A Metal-Based Biological Strategy for Hard Flexible Coatings. Science 2010, 328, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xue, K.; Loh, X.J. Thermo-Responsive Hydrogels: From Recent Progress to Biomedical Applications. Gels 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Jelken, J.; Jung, S.-H.; Lomadze, N.; Gordievskaya, Y.D.; Kramarenko, E.Y.; Pich, A.; Santer, S. Tuning the Volume Phase Transition Temperature of Microgels by Light. Adv. Funct. Mater. 2022, 32, 2107946. [Google Scholar] [CrossRef]
- Marcisz, K.; Jagleniec, D.; Mackiewicz, M.; Romanski, J.; Karbarz, M. Reversible and pH-modulated changes in microgel size triggered by electrochemical stimuli. Mater. Today Chem. 2022, 26, 101151. [Google Scholar] [CrossRef]
- Marcisz, K.; Zabost, E.; Karbarz, M. Electrosensitive polymer gels: Controlling size and shape by means of red–ox processes-outlook and prospects. Appl. Mater. Today 2022, 29, 101656. [Google Scholar] [CrossRef]
- Tokarev, I.; Orlov, M.; Katz, E.; Minko, S. An Electrochemical Gate Based on a Stimuli-Responsive Membrane Associated with an Electrode Surface. J. Phys. Chem. B 2007, 111, 12141–12145. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, M.; He, X.; Huang, L.; Zhang, Y.; Ren, B.; Zhong, M.; Chang, Y.; Yang, J.; Zheng, J. Dual Salt- and Thermoresponsive Programmable Bilayer Hydrogel Actuators with Pseudo-Interpenetrating Double-Network Structures. ACS Appl. Mater. Interfaces 2018, 10, 21642–21653. [Google Scholar] [CrossRef]
- Karbarz, M.; Mackiewicz, M.; Kaniewska, K.; Marcisz, K.; Stojek, Z. Recent Developments in Design and Functionalization of Micro and Nanostructural Environmentally-Sensitive Hydrogels Based on N-isopropylacrylamide. Appl. Mater. Today 2017, 9, 516–532. [Google Scholar] [CrossRef]
- Karbarz, M.; Gniadek, M.; Stojek, Z. One dimensional volume-phase transition of N-isopropylacrylamide gels on the surface of gold electrodes. Electroanalysis 2005, 17, 1396–1400. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Katz, E. Modified Electrodes and Electrochemical Systems Switchable by Temperature Changes. Electroanalysis 2016, 28, 1916–1929. [Google Scholar] [CrossRef]
- Hirotsu, S.; Hirokawa, Y.; Tanaka, T. Volume-phase transitions of ionized N-isopropylacrylamide gels. J. Chem. Phys. 1987, 87, 1392–1395. [Google Scholar] [CrossRef]
- Marcisz, K.; Romanski, J.; Stojek, Z.; Karbarz, M. Environmentally sensitive hydrogel functionalized with electroactive and complexing-iron(III) catechol groups. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3236–3242. [Google Scholar] [CrossRef]
- Karbarz, M.; Hyk, W.; Stojek, Z. Swelling ratio driven changes of probe concentration in pH- and ionic strength-sensitive poly(acrylic acid) hydrogels. Electrochem. Comm. 2009, 11, 1217–1220. [Google Scholar] [CrossRef]
- Marcisz, K.; Romanski, J.; Karbarz, M. Electroresponsive microgel able to form a monolayer on gold through self-assembly. Polymer 2021, 229, 123992. [Google Scholar] [CrossRef]
- Kaniewska, K.; Karbarz, M.; Stojek, Z. Electrochemical attachment of thermo- and pH sensitive interpenetrating-polymers-network hydrogel to conducting surface. Electrochim. Acta 2015, 179, 372–378. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, H.; Yan, K.; Li, X.; Lu, Z.; Wang, D. An ionic/thermal-responsive agar/alginate wet-spun microfiber-shaped hydrogel combined with grooved/wrinkled surface patterns and multi-functions. Carbohydr. Polym. 2023, 304, 120501. [Google Scholar] [CrossRef]
- Beaudoin, G.; Lasri, A.; Zhao, C.; Liberelle, B.; De Crescenzo, G.; Zhu, X.-X. Making Hydrophilic Polymers Thermoresponsive: The Upper Critical Solution Temperature of Copolymers of Acrylamide and Acrylic Acid. Macromolecules 2021, 54, 7963–7969. [Google Scholar] [CrossRef]
- Kaniewska, K.; Karbarz, M.; Ziach, K.; Siennicka, A.; Stojek, Z.; Hyk, W. Electrochemical Examination of the Structure of Thin Hydrogel Layers Anchored to Regular and Microelectrode Surfaces. J. Phys. Chem. B 2016, 120, 9540–9547. [Google Scholar] [CrossRef]
- Hisamatsu, N.; Iida, T.; Yasui, T.; Takada, K.; Yuchi, A. Double-side coated electrochemical actuator based on changes in volume of poly(acrylic acid) gel. Sens. Actuator B Chem. 2014, 203, 289–295. [Google Scholar] [CrossRef]
- Kaniewska, K.; Nowakowski, J.; Bacal, P.; Karbarz, M. Reversible change in volume of thin hydrogel layer deposited on electrode surface using Cu(II)↔Cu(I) process. Sens. Actuator B Chem. 2021, 344, 130114. [Google Scholar] [CrossRef]
- Marcisz, K.; Gawronska, A.; Stojek, Z.; Karbarz, M. Triggering the Shrinking/Swelling Process in Thin Gel Layers on Conducting Surfaces by Applying an Appropriate Potential. ACS Appl. Mater. Interfaces 2019, 11, 12114–12120. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Xie, M.; Jian, Y.; Wu, B.; Chen, C.; Zhao, C. Multiple-Responsive and Amphibious Hydrogel Actuator Based on Asymmetric UCST-Type Volume Phase Transition. ACS Appl. Mater. Interfaces 2019, 11, 43641–43648. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-X.; Zhao, X.-Y.; Jiang, J.-Q.; Liu, Z.-T.; Liu, Z.-W.; Li, G. Thermal-Responsive Hydrogel Actuators with Photo-Programmable Shapes and Actuating Trajectories. ACS Appl. Mater. Interfaces 2022, 14, 51244–51252. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Huang, G.; Cui, J.; Zhu, H.; Yan, G.; Mei, Y. Advances and Challenges of Hydrogel Materials for Robotic and Sensing Applications. Chem. Mater. 2022, 34, 9307–9328. [Google Scholar] [CrossRef]
- Shitanda, I.; Asano, R.; Hoshi, Y.; Itagaki, M.; Takada, K. An electrochemical actuator fabricated by transfer-printing of a carbon electrode onto a cupric-ion-containing poly(acrylic acid) gel surface. Electrochemistry 2020, 88, 236–239. [Google Scholar] [CrossRef]
- Takada, K.; Tanaka, N.; Tatsuma, T. A redox actuator based on reversible formation of bond between poly(acrylic acid) gel and Cu2+ ion. J. Electroanal. Chem. 2005, 585, 120–127. [Google Scholar] [CrossRef]
- Abdel-Hamid, R.; Newair, E.F. Electrochemical behavior of antioxidants: I. Mechanistic study on electrochemical oxidation of gallic acid in aqueous solutions at glassy-carbon electrode. J. Electroanal. Chem. 2011, 657, 107–112. [Google Scholar] [CrossRef]
- Novak, I.; Šeruga, M.; Komorsky-Lovrić, Š. Electrochemical Characterization of Epigallocatechin Gallate Using Square-Wave Voltammetry. Electroanalysis 2009, 21, 1003–1106. [Google Scholar] [CrossRef]
- Dubey, A.; Burke, N.A.D.; Stöver, H.D.H. Preparation and characterization of narrow compositional distribution polyampholytes as potential biomaterials: Copolymers of N-(3-aminopropyl)methacrylamide hydrochloride (APM) and methacrylic acid (MAA). J. Polym. Sci. Part A Polym. Chem. 2015, 53, 353–365. [Google Scholar] [CrossRef]
- Hyun, K.; Kim, S.H.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear as a way to classify the complex fluids. J. Non-Newton. Fluid Mech. 2002, 107, 51–65. [Google Scholar] [CrossRef]
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A review of nonlinear oscillatory shear tests: Analysis and application of large amplitude oscillatory shear (LAOS). Prog. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]
- Menyo, M.S.; Hawker, C.J.; Herbert Waite, J. Versatile tuning of supramolecular hydrogels through metal complexation of oxidation-resistant catechol-inspired ligands. Soft Matter 2013, 9, 10314–10323. [Google Scholar] [CrossRef]
- Holten-Andersen, N.; Harrington, M.J.; Birkedal, H.; Wait, J.H. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc. Natl. Acad. Sci. USA 2011, 108, 2651–2655. [Google Scholar] [CrossRef]
- Roberts, M.C.; Hanson, M.C.; Massey, A.P.; Karren, E.A.; Kiser, P.F. Dynamically Restructuring Hydrogel Networks Formedwith Reversible Covalent Crosslinks. Adv. Mater. 2007, 19, 2503–2507. [Google Scholar] [CrossRef]
- Charlet, A.; Lutz-Bueno, V.; Mezzenga, R.; Amstad, E. Shape retaining self-healing metal-coordinated hydrogels. Nanoscale 2021, 13, 4073–4084. [Google Scholar] [CrossRef]
- Zou, X.; Kui, X.; Zhang, R.; Zhang, Y.; Wang, X.; Wu, Q.; Chen, T.; Sun, P. Viscoelasticity and Structures in Chemically and Physically Dual-Cross-Linked Hydrogels: Insights from Rheology and Proton Multiple-Quantum NMR Spectroscopy. Macromolecules 2017, 50, 9340–9352. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaniewska, K.; Kościelniak, P.; Karbarz, M. pH Modulated Formation of Complexes with Various Stoichiometry between Polymer Network and Fe(III) in Thermosensitive Gels Modified with Gallic Acid. Gels 2023, 9, 447. https://doi.org/10.3390/gels9060447
Kaniewska K, Kościelniak P, Karbarz M. pH Modulated Formation of Complexes with Various Stoichiometry between Polymer Network and Fe(III) in Thermosensitive Gels Modified with Gallic Acid. Gels. 2023; 9(6):447. https://doi.org/10.3390/gels9060447
Chicago/Turabian StyleKaniewska, Klaudia, Patrycja Kościelniak, and Marcin Karbarz. 2023. "pH Modulated Formation of Complexes with Various Stoichiometry between Polymer Network and Fe(III) in Thermosensitive Gels Modified with Gallic Acid" Gels 9, no. 6: 447. https://doi.org/10.3390/gels9060447