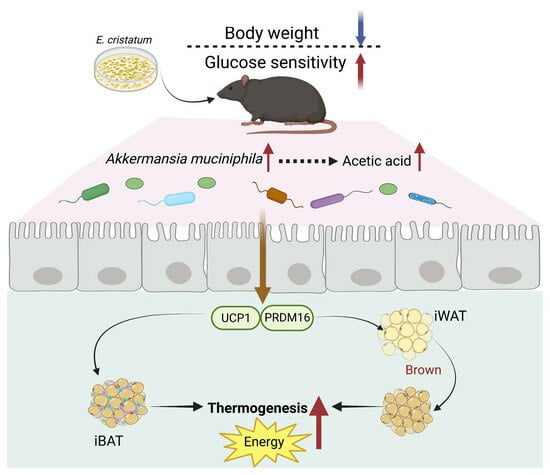
Eurotium cristatum from Fu Brick Tea Promotes Adipose Thermogenesis by Boosting Colonic Akkermansia muciniphila in High-Fat-Fed Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Experiments
2.3. Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
2.4. Analysis of Serum Biochemical Assessment
2.5. Examination of Histopathology and Immunofluorescence
2.6. qRT-PCR Analysis
2.7. Measurement of SCFAs
2.8. 16S rRNA Sequencing
2.9. Statistical Analysis
3. Results
3.1. Effects of E. cristatum on Body Weight and Glucolipid Metabolism in HFD-Fed Obese Mice
3.2. E. cristatum Promoted Thermogenesis in HFD-Fed Mice
3.3. E. cristatum Facilitated the Cecal SCFAs in HFD-Fed Mice
3.4. Impact of E. cristatum on Gut Microbiota Composition in HFD-Fed Obese Mice
3.5. Gut A. muciniphila Mediated E. cristatum-Induced Adipocyte Thermogenesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhao, Y.; Zhu, H.; Liang, N.; Ma, K.Y.; Lei, L.; He, W.S.; et al. Beneficial effects of tea water extracts on the body weight and gut microbiota in C57BL/6J mice fed with a high-fat diet. Food Funct. 2019, 10, 2847–2860. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; He, C.; Chen, Y.; Huang, Y.; Gao, Y.; Hou, A.Z.; Li, Y. UPLC-QQQ-MS/MS-based widely targeted metabolomic analysis reveals the effect of solid-state fermentation with Eurotium cristatum on the dynamic changes in the metabolite profile of dark tea. Food Chem. 2022, 378, 131999–132008. [Google Scholar] [CrossRef]
- Wang, X.L.; Liu, X.X.; Long, B.; Wei, F.Y.; Zhang, J.P.; Cui, Y.X. Comparison of chemical constituents of Eurotium cristatum-mediated pure and mixed fermentation in summer-autumn tea. LWT-Food Sci. Technol. 2021, 143, 111132. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, C.; Gong, J. Effects of enzymatic action on the formation of theabrownin during solid state fermentation of pu-erh tea. J. Sci. Food Agric. 2011, 91, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Jia, W. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 239–246. [Google Scholar]
- Wang, Y.; Zhao, A.; Du, H.; Liu, Y.; Qi, B.; Yang, X.B. Theabrownin from Fu Brick Tea Exhibits the Thermogenic Function of Adipocytes in High-Fat-Diet-Induced Obesity. J. Agric. Food Chem. 2021, 69, 11900–11911. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhong, K.; Bai, J.R.; Wu, Y.P.; Zhang, J.Q.; Gao, H. The biochemical characteristics of a novel fermented loose tea by Eurotium cristatum (mf800948) and its hypolipidemic activity in a zebrafish model–sciencedirect. LWT 2020, 117, 108629–108633. [Google Scholar] [CrossRef]
- Zhang, B.; Ren, D.; Zhao, A.; Cheng, Y.; Liu, Y.; Zhao, Y.; Yang, X. Eurotium cristatum reduces obesity by alleviating gut microbiota dysbiosis and modulating lipid and energy metabolism. J. Sci. Food Agric. 2022, 102, 7039–7051. [Google Scholar] [CrossRef]
- Tchkonia, T.; Thomou, T.; Zhu, Y.; Karagiannides, I.; Pothoulakis, C. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013, 17, 644–656. [Google Scholar]
- Sakers, A.; Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 10, 185. [Google Scholar]
- Queen, N.J.; Bates, R.; Huang, W.; Xiao, R.; Cao, L. Visceral Adipose Tissue-Directed FGF21 Gene Therapy Improves Metabolic and Immune Health in BTBR Mice. Mol. Ther. Methods Clin. Dev. 2020, 20, 110637–110645. [Google Scholar] [CrossRef] [PubMed]
- Nance, B.; Sierra, A.L.; Muir, J.; Lumeng, C. Adipose tissue macrophages: Regulators of adipose tissue immunometabolism during obesity. Mol. Metab. 2022, 66, 101642–101648. [Google Scholar] [CrossRef] [PubMed]
- Km, A.; Jg, A.; Pm, A.; Km, B.; Aka, B.; Mc, A. Mass spectrometry-based determination of lipids and small molecules composing adipose tissue with a focus on brown adipose tissue–sciencedirect. J. Pharmaceut. Biomed. 2020, 191, 18205–18213. [Google Scholar]
- Montanari, T.; Pošćić, N.; Colitti, M. Factors Involved in Whiteto-Brown Adipose Tissue Conversion and in Thermogenesis: A Review. Obes. Rev. 2017, 18, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Okamatsu-Ogura, Y.; Kuroda, M.; Tsutsumi, R.; Tsubota, R.; Saito, A.; Kimura, M. UCP1-dependent and UCP1-independent metabolic changes induced by acute cold exposure in brown adipose tissue of mice. Metabolism 2020, 113, 154396–154445. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Li, M.; Lam, S.M.; Wang, G.; Wu, Y.; Zhang, H.; Niu, C.; Zhang, X.; Liu, X.; et al. Microbiota Depletion Impairs Thermogenesis of Brown Adipose Tissue and Browning of White Adipose Tissue. Cell Rep. 2019, 26, 2720–2737. [Google Scholar]
- Kaisanlahti, A.; Glumoff, T. Browning of White Fat: Agents and Implications for Beige Adipose Tissue to Type 2 Diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Spiegelman, B.M. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–474. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Peng, C.; Liu, J. The phytochemical hyperforin triggers thermogenesis in adipose tissue via a Dlat-AMPK signaling axis to curb obesity. Cell Metab. 2021, 33, 565–580. [Google Scholar] [CrossRef]
- Betz, M.J.; Enerbäck, S. Targeting Thermogenesis in Brown Fat and Muscle to Treat Obesity and Metabolic Disease. Nat. Rev. Endocrinol. 2018, 14, 77–87. [Google Scholar]
- Saito, M.; Matsushita, M.; Yoneshiro, T. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11, 222–235. [Google Scholar]
- Richard, G.; Denis, P.B.; Syed, S.A.; Rossi, L. High-fructose feeding suppresses cold-stimulated brown adipose tissue glucose uptake independently of changes in thermogenesis and the gut microbiome. Cell Rep. Med. 2022, 9, 100742–100750. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.Y.; Zhao, C.N.; Xu, X.Y.; Tang, G.Y.; Li, H.B. Dietary plants, gut microbiota, and obesity: Effects and mechanisms. Trends Food Sci. Tech. 2019, 20, 194–204. [Google Scholar]
- Wu, J.; Zhang, B.; Liu, X.X.; Peng, L.; Liu, J.M.; Hu, Y.Z.; Ji, X.M. Current gut-on-a-chip platforms for clarifying the interactions between diet, gut microbiota, and host health. Trends Food Sci. Tech. 2023, 124, 1–12. [Google Scholar]
- Zhou, F.; Li, Y.L.; Zhang, X.; Wang, K.B.; Huang, J.A.; Liu, Z.H.; Zhu, M.Z. Polyphenols from Fu Brick Tea Reduce Obesity via Modulation of Gut Microbiota and Gut Microbiota-Related Intestinal Oxidative Stress and Barrier Function. J. Agric. Food Chem. 2021, 69, 14530–14543. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.M.; Zou, J.; Wu, M.; Peng, Z.J.; He, W.J.; Li, W.; Wu, Z.X. Noni (Morinda citrifolia L.) fruit polysaccharide ameliorated high-fat diet-induced obesity by modulating gut microbiota and improving bile acid metabolism. J. Funct. Foods 2023, 101, 105408. [Google Scholar]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Kim, J.Y.; Han, D.Y. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef]
- Lee, P.S.; Lu, Y.Y.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Calebin-A prevents HFD-induced obesity in mice by promoting-thermogenesis and modulating gut microbiota. J. Tradit. Complement. Med. 2023, 13, 119–127. [Google Scholar] [CrossRef]
- Du, H.P.; Wang, Q.; Xingbin, Y. Fu brick tea alleviates chronic kidney disease of rats with high fat diet consumption through attenuating insulin resistance in skeletal muscle. J. Agric. Food Chem. 2019, 67, 2839–2847. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Liu, Y.Y.; Yang, C.C.; Liu, L.; Zhang, X.N.; Yang, X.B. Heimao Tea Polysaccharides Ameliorate Obesity via Enhancing Gut Microbiota-Dependent Adipocytes Thermogenesis in Mice Fed with High Fat Diet. Food Funct. 2022, 13, 13014–13027. [Google Scholar] [CrossRef]
- Wong, K.H.; Tsoi, O.; Huang, F.Y.; Seto, W.K.; Fung, J. Application of coamplification at lower denaturation temperature-PCR sequencing for early detection of antiviral drug resistance mutations of hepatitis b virus. J. Clin. Microbiol. 2014, 52, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xia, J.; Liang, Q.; Wang, Y.; Wang, Y.; Hu, P.; Li, P.; Luo, G. Plasma esterified and non-esterified fatty acids metabolic profiling using gas chromatography-mass spectrometry and its application in the study of diabetic mellitus and diabeticnephropathy. Anal. Chim. Acta 2011, 689, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Marie, S.P.; Gius, C.; Claudia, V.; Sebastian, A.; Yommine, H.; Johan, D.; Gabriele, R.; Rikard, L. The human gut microbiota and glucose metabolism: A scoping review of key bacteria and the potential role of SCFAs. Am. J. Clin. Nutr. 2022, 116, 862–874. [Google Scholar]
- Zhang, C.Y.; Guo, J.; Zhang, Z.; Tian, S.; Liu, Z.H. Biochemical components and fungal community dynamics during the flowering process of Moringa-Fu brick tea, a novel microbially fermented blended tea. LWT 2021, 140, 110822–110831. [Google Scholar]
- Chen, Q.; Zhang, M.; Chen, M.; Li, M.R.; Yue, P.X. Influence of Eurotium cristatum and Aspergillus niger individual and collaborative inoculation on volatile profile in liquid-state fermentation of instant dark teas. Food Chem. 2021, 350, 129234–129240. [Google Scholar]
- Wu, H.; Zhao, H.H.; Ding, J.; Wang, Y.H.; Jian, H.; Yang, L. Metabolites and microbial characteristics of Fu brick tea after natural fermentation. LWT 2023, 181, 114775–114780. [Google Scholar]
- Guo, B.; Liu, B.; Wei, H.; Chen, F. Front cover: Extract of the microalga nitzschia laevis prevents high-fat-diet-induced obesity in mice by modulating the composition of gut microbiota. Mol. Nutr. Food Res. 2019, 63, 1800808. [Google Scholar]
- Sung, M.M.; Kim, T.T.; Denou, E.; Soltys, C.M.; Hamza, S.M.; Byrne, N.J.; Madsen, K. Improved glucose homeostasis in obese mice treated with resveratrol is associated with alterations in the gut microbiome. Diabetes 2017, 66, 418–425. [Google Scholar]
- Mu, H.; Zhou, Q.; Yang, R.; Zeng, J.; Dong, J. Naringin attenuates high fat diet induced non-alcoholic fatty liver disease and gut bacterial dysbiosis in mice. Front. Microbiol. 2020, 11, 585066. [Google Scholar] [CrossRef]
- Wang, Q.; Li, D.; Cao, G.; Shi, Q.; Zhu, J. Il-27 signalling promotes adipocyte thermogenesis and energy expenditure. Nature 2021, 600, 314–318. [Google Scholar]
- Han, S.J.; Zaretsky, A.G.; Andrade-Oliveira, V.; Dzutsev, A. White adipose tissue is a reservoir for memory t cells and promotes protective memory responses to infection. Immunity 2017, 47, 1154–1168. [Google Scholar]
- Wu, Q.; Liang, X.Y.; Wang, K.; Lin, J.; Wang, X.M.; Wang, P.C.; Zhang, Y.M.; Nie, Q.X.; Liu, H.Y.; Liu, J.H.; et al. Intestinal hypoxia-inducible factor 2α regulates lactate levels to shape the gut microbiome and alter thermogenesis. Cell Metab. 2021, 33, 1988–2003. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Tan, B.K.; Christiand, M. Dietary polyphenols turn fat “brown”: A narrative review of the possible mechanisms. Trends Food Sci. Tech. 2020, 97, 221–232. [Google Scholar]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-Chain Fatty Acids Stimulate Leptin Production in Adipocytes through the G Protein-Coupled Receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.F.; Dong, H.; Balaz, M.; Slyper, M.; Georgia, C.; Antonio, G.; Zuzana, K.; Patrik, S.; Lucia, B.; Ding, L.; et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 2020, 587, 98–102. [Google Scholar] [PubMed]
- Minamino, K.; Nagasawa, Y.; Ohtsuru, M. A water-soluble extract from Grifola frondosa, maitake mushroom, decreases lipid droplets in brown adipocyte tissue cells. J. Nutr. Sci. Vitaminol. 2008, 54, 497–500. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Wang, B.; Su, M.; Zhou, S. Prevention and control effects of edible fungi and their active ingredients on obesity: An updated review of research and mechanism. J. Funct. Foods 2023, 107, 105621–105630. [Google Scholar]
- Du, H.; Shi, L.; Wang, Q.; Yan, T.; Wang, Y.; Yang, X.B. Fu brick tea protects against high-fat diet-inducedobesity phenotypes via promoting adipose browning and thermogenesis in association with gut microbiota. Food Funct. 2022, 13, 11111–11120. [Google Scholar] [CrossRef]
- Du, H.; Shi, L.; Wang, Q.; Yan, T.; Wang, Y.; Yang, X.B. Fu Brick Tea Polysaccharides Prevent Obesity via Gut Microbiota-Controlled Promotion of Adipocyte Browning and Thermogenesis. J. Agric. Food Chem. 2022, 70, 13893–13903. [Google Scholar] [CrossRef]
- Chien, Y.H.; Yu, H.Y.; Hsiang, Y.; Chen, Y.W. Taiwanese green propolis ameliorates metabolic syndrome via remodeling of white adipose tissue and modulation of gut microbiota in diet-induced obese mice. Biomed. Pharmacother. 2023, 160, 114386–114395. [Google Scholar]
- Zou, T.; Xie, F.; Liang, P.B.; Chen, J.; Wang, Z.; Du, M.; You, J. Polysaccharide-rich fractions from Enteromorpha prolifera improve hepatic steatosis and gut barrier integrity in high-fat diet-induced obese mice linking to modulation of gut microbiota. Biomed. Pharmacother. 2023, 157, 114034–114040. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van, H.M.; Vieira, S.S.; Falony, G.; Raes, J. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Cani, P.D.; Mde, W.V. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C. Function of Akkermansia muciniphila in obesity: Interactions with lipid metabolism, immune response and gut systems. Front. Microbiol. 2020, 11, 219. [Google Scholar]
- Cheng, D.; Xie, M.Z. A review of a potential and promising probiotic candidate—Akkermansia muciniphila. J. Appl. Microbiol. 2021, 130, 1813–1822. [Google Scholar] [PubMed]
- Ma, J.; Liu, Z.; Gao, X.X.; Bao, Y.H.; He, X.F.; Li, Y.; Huang, W.J.; Chen, H.Z.; Li, H.K. Gut microbiota remodeling improves natural aging-related disorders through Akkermansia muciniphila and its derived acetic acid. Pharmacol. Res. 2023, 189, 106687–106690. [Google Scholar] [PubMed]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y. Scutellaria baicalensis georgi polysaccharide ameliorates dss-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2020, 166, 1035–1045. [Google Scholar]








| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Pgc1α | GTGTGTGCTGTGTGTCAGAG | AACCAGAGCAGCACACTCTAT |
| Prdm16 | CATGTGCGAAGGTGTCCAAA | GTCACCGTCACTTTTGGCT |
| Pparγ | GACGCGGAAGAAGAGACCTG | TCACCGCTTCTTTCAAATCTTGT |
| Ucp1 | GTGAACCCGACAACTTCCGA | TGGCCTTCACCTTGGATCTGA |
| Cidea | AGGCCGTGTTAAGGAATCTGC | AACCAGVVTTTGGTGCTAGG |
| Nrf1 | CTGCAGGTCCTGTGGGAAT | GGCTCTGAGTTTCCGAAGCA |
| Nrf2 | AGGCCGTGTTAAGGAATCTGC | TATCCAGGGCAAGCGACTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, T.; Yang, C.; Wu, Y.; Liu, Y.; Yang, X. Eurotium cristatum from Fu Brick Tea Promotes Adipose Thermogenesis by Boosting Colonic Akkermansia muciniphila in High-Fat-Fed Obese Mice. Foods 2023, 12, 3716. https://doi.org/10.3390/foods12203716
Wang Y, Li T, Yang C, Wu Y, Liu Y, Yang X. Eurotium cristatum from Fu Brick Tea Promotes Adipose Thermogenesis by Boosting Colonic Akkermansia muciniphila in High-Fat-Fed Obese Mice. Foods. 2023; 12(20):3716. https://doi.org/10.3390/foods12203716
Chicago/Turabian StyleWang, Yu, Ting Li, Chengcheng Yang, Yingmei Wu, Yueyue Liu, and Xingbin Yang. 2023. "Eurotium cristatum from Fu Brick Tea Promotes Adipose Thermogenesis by Boosting Colonic Akkermansia muciniphila in High-Fat-Fed Obese Mice" Foods 12, no. 20: 3716. https://doi.org/10.3390/foods12203716