Carbon-Supported KCoMoS2 for Alcohol Synthesis from Synthesis Gas
Abstract
:1. Introduction
2. Results
2.1. Characterization
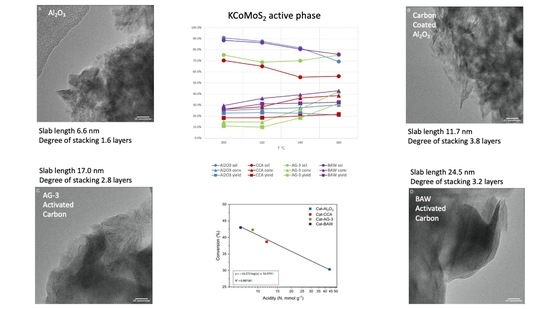
2.1.1. Elemental Analysis and KCoMoS2 Particle Size
2.1.2. Textural Characteristics
2.1.3. Acidity
2.2. Catalytic Experiments
3. Discussion
4. Materials and Methods
4.1. Preparation of Catalysts
4.2. Physical Characterization
4.2.1. Textural Properties
4.2.2. Elemental Composition
4.2.3. Acidic Properties
4.3. Catalytic Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, K.L.; Menard, R.J.; English, B.C. Market Analysis for Fischer-Tropsch waxes. Chem. Eng. Trans. 2014, 57, 1669–1674. [Google Scholar]
- De Klerk, A. Fischer-Tropsch Refining; Wiley-VCH Verlag GmbH & Co.: Berlin, Germany, 2011. [Google Scholar]
- Beretta, A.; Sun, Q.; Herman, R.G.; Klier, K. Production of Methanol and Isobutyl Alcohol Mixtures over Double-Bed Cesium-Promoted Cu/ZnO/Cr2O3 and ZnO/Cr2O3 Catalysts. Ind. Eng. Chem. Res. 1996, 35, 1534–1542. [Google Scholar] [CrossRef]
- Brown, R.C. Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power; Wiley: Chichester, UK, 2011. [Google Scholar]
- Suarez Paris, R.; Lopez, L.; Baronet, J.; Pardo, F.; Botoonet, M.; Jaras, S. Catalytic conversion of biomass-derived synthesis gas to fuels. Catalysis 2015, 27, 62–143. [Google Scholar]
- Surisetty, V.R.; Tavasoli, A.; Dalai, A.K. Synthesis of higher alcohols from syngas over alkali promoted MoS2 catalysts supported on multi-walled carbon nanotubes. Appl. Catal. A Gen. 2009, 365, 243–251. [Google Scholar] [CrossRef]
- Zaman, S.; Smith, K.J. A Review of Molybdenum Catalysts for Synthesis Gas Conversion to Alcohols: Catalysts, Mechanisms and Kinetics. Catal. Rev. 2012, 54, 41–132. [Google Scholar] [CrossRef]
- Luk, H.T.; Mondelli, C.; Ferré, D.C.; Stewart, J.A.; Pérez-Ramírez, J. Status and prospects in higher alcohols synthesis from syngas. Chem. Soc. Rev. 2017, 46, 1358–1426. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, E.A.; Dorokhov, V.S.; Maximov, V.V.; Nikulshin, P.A.; Pimerzin, A.A.; Kogan, V.M. Computational and experimental study of the second metal effect on the structure and properties of bi-metallic MeMoS-sites in transition metal sulfide catalysts. Catal. Today 2018, 305, 19–27. [Google Scholar] [CrossRef]
- Dorokhov, V.S.; Ishutenko, D.I.; Nikul’shin, P.A.; Kotsareva, K.V.; Trusova, E.A.; Bondarenko, T.N.; Eliseev, O.L.; Lapidus, A.L.; Rozhdestvenskaya, N.N.; Kogan, V.M. Conversion of Synthesis Gas into Alcohols on Supported Cobalt– Molybdenum Sulfide Catalysts Promoted with Potassium. Kinet. Catal. 2013, 54, 253–262. [Google Scholar] [CrossRef]
- Surisetty, V.R.; Dalai, A.K.; Kozinski, J. Intrinsic Reaction Kinetics of Higher Alcohol Synthesis from Synthesis Gas over Sulfided Alkali-promoted Co-Rh-Mo Trimetallic Catalyst Supported on MWCNTs. Energy Fuels 2010, 24, 4130–4137. [Google Scholar] [CrossRef]
- Song, C.L.; Zhang, W.M.; Pei, Y.Q.; Fan, G.L.; Xu, G.P. Comparative effects of MTBE and ethanol additions into gasoline on exhaust emissions. Atmos. Environ. 2006, 40, 1957–1970. [Google Scholar] [CrossRef]
- Bergthorson, J.M.; Thomson, M.J. A review of the combustion and emissions properties of advanced transportation biofuels and their impact on existing and future engines. Renew. Sustain. Energy Rev. 2015, 42, 1393–1417. [Google Scholar] [CrossRef]
- Christensen, J.M.; Mortensen, P.M.; Trane, R.; Jensen, P.A.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2009, 366, 29–43. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Y.; Bao, J.; Jiang, M.; Hu, T.; Liu, T.; Xie, Y. Effect of Cobalt promoter on Co-Mo-K/C catalysts used for mixed alcohol synthesis. Appl. Catal. A Gen. 2001, 220, 21–30. [Google Scholar] [CrossRef]
- Kogan, V.M.; Nikulshin, P.A. On the dynamic model of promoted molybdenum sulfide catalysts. Catal. Today 2010, 149, 224–231. [Google Scholar] [CrossRef]
- Duchet, J.C.; van Oers, E.M.; de Beer, V.H.J.; Prins, R. Carbon-Supported Sulfide Catalysts. J. Catal. 1983, 80, 386–402. [Google Scholar] [CrossRef]
- Marsh, H.; Rodriguez-Reinoso, F. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–554. [Google Scholar]
- Kohl, A.; Linsmeier, C.; Taglauer, E.; Knozinger, H. Influence of Support and Promotor on the. Catalytic Activity of Rh/VOx/SiO2 Model Catalysts. Phys. Chem. 2001, 3, 4639–4643. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Baker, R.T.K.; Horsley, J.A. Strong Interactions in Supported-Metal Catalysts. Science 1981, 211, 1121–1125. [Google Scholar] [CrossRef] [Green Version]
- Hindermann, J.P.; Deluzarxhe, A.; Kieffer, R.; Kiennemann, A. Characterization of Chemisorbed Species in CO/H2 and CO2/H2 Reactions: Evolutive Behaviour of the Species. Can. J. Chem. Eng. 1983, 61, 21–28. [Google Scholar] [CrossRef]
- Li, D.; Li, R.; Lu, M.; Lin, X.; Zhan, Y.; Jiang, L. Carbon dioxide reforming of methane over Ru catalysts supported on Mg-Al oxides: A highly dispersed and stable Ru/Mg(Al)O catalyst. Appl. Catal. B Environ. 2017, 200, 566–577. [Google Scholar] [CrossRef]
- Lva, M.; Xie, W.; Sun, S.; Wu, G.; Zheng, L.; Chu, S.; Gao, C.; Bao, J. Activated-carbon-supported K-Co–Mo catalyst for synthesis of higher alcohols from syngas. Catal. Sci. Technol. 2015, 5, 2925–2934. [Google Scholar] [CrossRef]
- Dorokhov, V.S.; Kamorin, M.A.; Rozhdestvenskaya, N.N.; Kogan, V.M. Synthesis and conversion of alcohols over modified transition metal sulphides. Comptes Rendus Chim. 2016, 19, 1184–1193. [Google Scholar] [CrossRef]
- Mukhin, V.M.; Klushin, V.N. Production and Application of Carbon Adsorbents; Zhodyakina, N.F., Ed.; Russian Mendeleev University of Chemical Technology of Russia: Moscow, Russia, 2012. (In Russian) [Google Scholar]
- Sing, K.S.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Claure, M.T.; Chai, S.H.; Dai, S.; Unocic, K.A.; Alamgir, F.M.; Agrawal, P.K.; Jones, C.W. Tuning of higher alcohol selectivity and productivity in CO hydrogenation reactions over K/MoS2 domains supported on mesoporous activated carbon and mixed MgAl oxide. J. Catal. 2015, 97, 324–388. [Google Scholar]
- Morrill, M.R.; Thao, N.T.; Shou, H.; Davis, R.J.; Barton, D.J.; Ferrari, D.; Agrawal, P.K.; Jones, C.W. Origins of unusual alcohol selectivities over mixed MgAl oxide supported K/MoS2 catalysts for higher alcohol synthesis from syngas. ACS Catal. 2016, 3, 1665–1675. [Google Scholar] [CrossRef]
- Maximov, V.V.; Permyakov, E.; Dorokhov, V.A.; Wang, Y.; Kooyman, P.J.; Kogan, V.M. Effect of Promoter Nature on Synthesis Gas Conversion to Alcohols over (K)MeMoS2 /Al2O3 Catalysts. ChemCatChem 2020, 12, 1443–1452. [Google Scholar] [CrossRef]
- Nikulshin, P.A.; Salnikov, V.A.; Mozhaev, A.V.; Minaev, P.P.; Kogan, V.M.; Pimerzin, A.A. Relationship between active phase morphology and catalytic properties of the carbon–alumina-supported Co(Ni)Mo catalysts in HDS and HYD reactions. J. Catal. 2014, 309, 386–396. [Google Scholar] [CrossRef]
- Toyoda, T.; Minami, T.; Qian, E.W. Mixed alcohol synthesis over sulfided molybdenum-based catalysts energy. Fuels 2013, 27, 3769–3777. [Google Scholar] [CrossRef]
- Zurita, M.J.P.; Cifarelli, M.; Cubeiro, M.L.; Goldwasser, J.A.M.; Pietri, E.; Garcia, L.; Aboukais, A.; Lamonier, J.-F. Palladium-based Catalysts for the Synthesis of Alcohols. J. Mol. Catal. A Chem. 2003, 206, 339–351. [Google Scholar] [CrossRef]
- Lee, J.J.; Han, S.; Kim, H.; Koh, J.H.; Hyeon, T.; Moon, S.H. Performance of CoMoS catalysts supported on nanoporous carbon in the hydrodesulfurization of dibenzothiophene and 4,6-dimethyldibenzothiophene. Catal. Today 2003, 194, 86–141. [Google Scholar] [CrossRef]
- Anashkin, Y.A.; Ishutenko, D.I.; Maximov, V.; Pimerzin, A.; Kogan, V.M.; Nikulshin, P.A. Effect of carrier properties on the activity of supported KCoMoS catalysts in the synthesis of alcohol from syngas. React. Kinet. Mech. Catal. 2019, 5, 11335–11347. [Google Scholar] [CrossRef]
- Surisetty, V.R.; Eswaramoorthi, I.; Dalai, A.K. Comparative study of higher alcohols synthesis over alumina and activated carbon-supported alkali-modified MoS2 catalysts promoted with group VIII metals. Fuel 2012, 96, 77–84. [Google Scholar] [CrossRef]
- Surisetty, V.R.; Dalai, A.K.; Kozinski, J. Influence of porous characteristics of the carbon support on alkali-modified trimetallic Co–Rh–Mo sulfided catalysts for higher alcohols synthesis from synthesis gas. Appl. Catal. A Gen. 2011, 393, 50–58. [Google Scholar] [CrossRef]




| Content (wt%) | Molar Ratio | Average Slab | |||||
|---|---|---|---|---|---|---|---|
| Catalyst | Mo | K | Co | r 1 | t 2 | Length (nm) | Stacking (Layers) |
| KCoMoS2/Al2O3 | 15.8 | 10.2 | 5.1 | 0.34 | 1.03 | 6.6 | 1.6 |
| KCoMoS2/CCA | 14.4 | 11.6 | 4.7 | 0.34 | 1.30 | 11.7 | 3.8 |
| KCoMoS2/AG-3 | 14.9 | 12.3 | 5.7 | 0.39 | 1.24 | 17.0 | 2.8 |
| KCoMoS2/BAW | 14.8 | 12.0 | 5.2 | 0.36 | 1.27 | 24.5 | 3.2 |
| Sample | Stotal, m2/g | Smicro, m2/g | Smeso 1, m2/g | Vtotal, cm3/g | Vmicro, cm3/g | Vmeso 2, cm3/g | Acidity 3, mmol/g |
|---|---|---|---|---|---|---|---|
| Al2O3 | 161 | 0 | 161 | 0.65 | 0.00 | 0.65 | 286 |
| KCoMoS2/Al2O3 | 91 | 0 | 91 | 0.29 | 0.00 | 0.29 | 44 |
| CCA | 156 | 13 | 143 | 0.63 | 0.01 | 0.63 | 4 |
| KCoMoS2/CCA | 73 | 0 | 73 | 0.26 | 0.00 | 0.26 | 12 |
| AG-3 | 854 | 753 | 101 | 0.45 | 0.35 | 0.10 | 4 |
| KCoMoS2/AG-3 | 164 | 137 | 27 | 0.09 | 0.06 | 0.03 | 9 |
| BAW | 753 | 642 | 111 | 0.39 | 0.26 | 0.13 | 0 |
| KCoMoS2/BAW | 404 | 365 | 40 | 0.23 | 0.16 | 0.07 | 7 |
| Catalyst | GHSV h−1 | p MPa | T °C | Conv % | Tot Liq Sel % | Ref |
|---|---|---|---|---|---|---|
| KCoMoS2/Al2O3 | 760 | 5 | 340 | 23 | 48 | [29] |
| KCoMoS2/CCA | 760 | 5 | 340 | 19.2 | 65 | [25] |
| KCoMoS2/MWCNT | 1200 | 8.3 | 320 | 25 | 40 | [6] |
| KCoMoS2/AC-CGP 1 | 1200 | 8.3 | 330 | 44.5 | 27.5 | [30] |
| KCoMoS2/AC-RX3 2 | 1200 | 8.3 | 330 | 39.6 | 25.8 | [30] |
| KCoMoS2/AC-Darco 3 | 1200 | 8.3 | 330 | 35.6 | 24.8 | [30] |
| KCoMoS2/Al2O3 | 760 | 5 | 360 | 30.3 | 69.2 | current 4 |
| KCoMoS2/CCA | 760 | 5 | 360 | 36.4 | 71.2 | current 4 |
| KCoMoS2/AG-3 | 760 | 5 | 360 | 42.3 | 75.1 | current 4 |
| KCoMoS2/BAW | 760 | 5 | 360 | 43.0 | 75.7 | current 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, M.E.; Maximov, V.V.; Dorokhov, V.S.; Mukhin, V.M.; Sheshko, T.F.; Kooyman, P.J.; Kogan, V.M. Carbon-Supported KCoMoS2 for Alcohol Synthesis from Synthesis Gas. Catalysts 2021, 11, 1321. https://doi.org/10.3390/catal11111321
Osman ME, Maximov VV, Dorokhov VS, Mukhin VM, Sheshko TF, Kooyman PJ, Kogan VM. Carbon-Supported KCoMoS2 for Alcohol Synthesis from Synthesis Gas. Catalysts. 2021; 11(11):1321. https://doi.org/10.3390/catal11111321
Chicago/Turabian StyleOsman, Mohamed E., Vladimir V. Maximov, Viktor S. Dorokhov, Viktor M. Mukhin, Tatiana F. Sheshko, Patricia J. Kooyman, and Viktor M. Kogan. 2021. "Carbon-Supported KCoMoS2 for Alcohol Synthesis from Synthesis Gas" Catalysts 11, no. 11: 1321. https://doi.org/10.3390/catal11111321