High-Resolution Airborne Hyperspectral Imagery for Assessing Yield, Biomass, Grain N Concentration, and N Output in Spring Wheat
Abstract
:1. Introduction
2. Materials and Methods
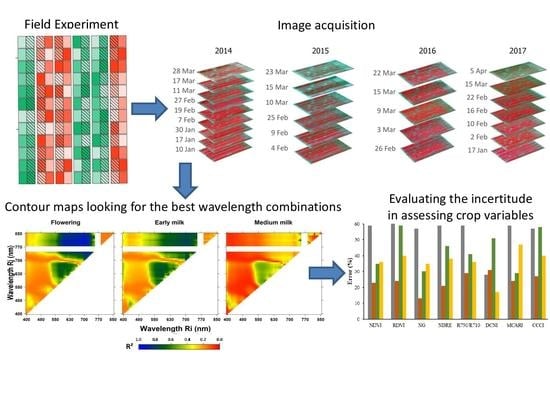
2.1. Field Experiment and Crop Data Collection
2.2. Hyperspectral Flight Campaign and Data Processing
2.3. Spectral Exploratory Analysis Using Narrow-Band Combinations
2.4. Spectral Indices and Assessment of Crop Variables
2.5. Application to N Fertilizer Recommendation
2.6. Statistical Analysis
3. Results
3.1. Agronomic Data
3.1.1. Grain Yield and Biomass
3.1.2. Grain N Concentration and N Output
3.2. Spectral Signatures of the Treatments
3.3. Spectral Exploratory Analysis Using Narrow-Band Combinations
3.3.1. Grain Yield and Biomass
3.3.2. Grain N Concentration and N Output
3.4. Spectral Indices and Assessment of Crop Variables
3.4.1. Grain Yield and Biomass
3.4.2. Grain N Concentration and N Output
3.5. Application to N Fertilizer Recommendation for Increasing GNC
4. Discussion
4.1. What Are the Best Indices for Estimating GY, Biomass, GNC, and N Output
4.2. When Is the Best Growth Stage for Assessing GY, Biomass, GNC, and N Output
4.3. Use of Spectral Information to Guide Fertilizer Recommendation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matson, P.A.; Naylor, R.; Ortiz-Monasterio, I. Integration of environmental, agronomic, and economic aspects of fertilizer management. Science 1998, 280, 112–115. [Google Scholar] [CrossRef] [Green Version]
- Arregui, L.M.; Quemada, M. Strategies to improve nitrogen-use efficiency in winter cereal crops under rainfed Mediterranean conditions. Agron. J. 2008, 100, 277–284. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. World Fertilizer Trends and Outlook to 2019; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, J.; Matson, P. Patterns and controls of nitrous oxide emissions from drainage waters of the Yaqui Valley, Sonora, Mexico. Glob. Biogeochem. Cycles 2003, 17, 1080. [Google Scholar] [CrossRef]
- Beman, J.M.; Arrigo, K.R.; Matson, P.A. Agricultural runoff fuels large phytoplankton blooms in vulnerable areas of the ocean. Nature 2005, 434, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Colaço, A.F.; Bramley, R.G.V. Site–Year Characteristics Have a Critical Impact on Crop Sensor Calibrations for Nitrogen Recommendations. Agron. J. 2019, 111, 2047–2059. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Gitelson, A.A.; Schepers, J.S.; Walthall, C.L. Application of spectral remote sensing for agronomic decisions. Agron. J. 2008, 100, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Hansen, P.M.; Schjoerring, J.K. Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens. Environ. 2003, 86, 542–553. [Google Scholar] [CrossRef]
- Prey, L.; Schmidhalter, U. Simulation of satellite reflectance data using high-frequency ground based hyperspectral canopy measurements for in-season estimation of grain yield and grain nitrogen status in winter wheat. ISPRS J. Photogramm. Remote Sens. 2019, 149, 176–187. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Zarco-Tejada, P.J.; López-Herrera, P.J.; Pérez-Martín, E.; Alonso-Ayuso, M.; Quemada, M. Airborne and ground level sensors for monitoring nitrogen status in a maize crop. Biosyst. Eng. 2017, 160, 124–133. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Save, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Fox, R.H.; Walthall, C.L. Crop Monitoring Technologies to Assess Nitrogen Status. In Nitrogen in Agricultural Systems, Agronomy Monograph 49; Schepers, J.S., Raun, W.R., Eds.; ASA, CSSA, SSSA: Madison, WI, USA, 2008; pp. 647–674. [Google Scholar] [CrossRef]
- Fischer, R.A.; Howe, G.N.; Ibrahim, Z. Irrigated spring wheat and timing and amount of nitrogen fertilizer. I. Grain yield and protein content. Field Crop. Res. 1993, 33, 37–56. [Google Scholar] [CrossRef]
- Ravier, C.; Quemada, M.; Jeuffroy, M.H. Use of a chlorophyll meter to assess nitrogen nutrition index during the growth cycle in winter wheat. Field Crop. Res. 2017, 214, 73–82. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Stone, M.L.; Martin, K.L.; Freeman, K.W.; Mullen, R.W.; Zhang, H.; Schepers, J.S.; Johnson, G.V. Optical sensor-based algorithm for crop nitrogen fertilization. Commun. Soil Sci. Plant Anal. 2005, 36, 2759–2781. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Miao, Y.; Zhang, F.; Cui, Z.; Li, R.; Chen, X.; Zhang, H.; Schroder, J.; Raun, W.R.; Jia, L. In-season optical sensing improves nitrogen-use efficiency for winter wheat. Soil Sci. Soc. Am. J. 2009, 73, 1566–1574. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index—the canopy chlorophyll content index (CCCI). Field Crop. Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Li, F.; Mistele, B.; Hu, Y.; Yue, X.; Yue, S.; Miao, Y.; Chen, X.; Cui, Z.; Meng, Q.; Schmidhalter, U. Remotely estimating aerial N status of phenologically differing winter wheat cultivars grown in contrasting climatic and geographic zones in China and Germany. Field Crop. Res. 2012, 138, 21–32. [Google Scholar] [CrossRef]
- Diacono, M.; Rubino, P.; Montemurro, F. Precision nitrogen management of wheat. A review. Agron. Sustain. Dev. 2013, 33, 219–241. [Google Scholar] [CrossRef]
- Babar, M.A.; van Ginkel, M.; Klatt, A.R.; Prasad, B.; Reynolds, M.P. The potential of using spectral reflectance indices to estimate yield in wheat grown under reduced irrigation. Euphytica 2006, 150, 155–172. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Blasch, G.; Defourny, P.; Ortiz-Monasterio, J.I.; Schulthess, U.; Zarco-Tejada, P.J.; Taylor, J.A.; Gérard, B. Multi-temporal and spectral analysis of high-resolution hyperspectral airborne imagery for precision agriculture: Assessment of wheat grain yield and grain protein content. Remote Sens. 2018, 10, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziadi, N.; Bélanger, G.; Claessens, A.; Lefebvre, L.; Cambouris, A.N.; Tremblay, N.; Nolin, M.C.; Parent, L.É. Determination of a critical nitrogen dilution curve for spring wheat. Agron. J. 2010, 102, 241–250. [Google Scholar] [CrossRef]
- Magney, T.S.; Eitel, J.U.H.; Huggins, D.R.; Vierling, L.A. Proximal NDVI derived phenology improves in-season predictions of wheat quantity and quality. Agric. For. Meteorol. 2016, 217, 46–60. [Google Scholar] [CrossRef]
- Prey, L.; Schmidhalter, U. Deep Phenotyping of Yield-Related Traits in Wheat. Agronomy 2020, 10, 603. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.M.; McBratney, A.B.; Skerritt, J.H. Site-specific durum wheat quality and its relationship to soil properties in a single field in northern New South Wales. Precis. Agric. 2002, 3, 155–168. [Google Scholar] [CrossRef]
- Ottman, M.J.; Doerge, T.A.; Martin, E.C. Durum grain quality as affected by nitrogen fertilization near anthesis and irrigation during grain fill. Agron. J. 2000, 92, 1035–1041. [Google Scholar] [CrossRef]
- Martre, P.; Porter, J.R.; Jamieson, P.D.; Triboï, E. Modeling grain nitrogen accumulation and protein composition to understand the sink/source regulations of nitrogen remobilization for wheat. Plant Physiol. 2003, 133, 1959–1967. [Google Scholar] [CrossRef] [Green Version]
- Rharrabti, Y.; Villegas, D.; Garcia Del Moral, L.F.; Aparicio, N.; Elhani, S.; Royo, C. Environmental and genetic determination of protein content and grain yield in durum wheat under Mediterranean conditions. Plant Breed. 2001, 120, 381–388. [Google Scholar] [CrossRef]
- Magney, T.S.; Eitel, J.U.; Vierling, L.A. Mapping wheat nitrogen uptake from RapidEye vegetation indices. Precis. Agric. 2017, 18, 429–451. [Google Scholar] [CrossRef]
- Bonfil, D.J.; Karnieli, A.; Raz, M.; Mufradi, I.; Asido, S.; Egozi, H.; Hoffman, A.; Schmilovitch, Z. Decision support system for improving wheat grain quality in the Mediterranean area of Israel. Field Crop. Res. 2004, 89, 153–163. [Google Scholar] [CrossRef]
- Gil, D.H.; Bonfil, D.J.; Svoray, T. Multi scale analysis of the factors influencing wheat quality as determined by Gluten Index. Field Crop. Res. 2011, 123, 1–9. [Google Scholar] [CrossRef]
- Long, D.S.; Engel, R.E.; Siemens, M.C. Measuring grain protein concentration with in-line near infrared reflectance spectroscopy. Agron. J. 2008, 100, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.B.; Asner, G.P.; Ortiz-Monasterio, J.I.; Benning, T.L. Remote sensing of regional crop production in the Yaqui Valley, Mexico: Estimates and uncertainties. Agri. Ecosyst. Environ. 2003, 94, 205–220. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Garcia, J.; Almendros, P.; Quemada, M. Ground cover and leaf area index relationship in a grass, legume and crucifer crop. Plant Soil Environ. 2012, 58, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Monasterio, J.I.; Sayre, K.D.; Rajaram, S.; McMahon, M. Genetic progress in wheat yield and nitrogen use efficiency under four nitrogen rates. Crop Sci. 1997, 37, 898–904. [Google Scholar] [CrossRef]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; van Kessel, C. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar] [CrossRef]
- Latshaw, S.P.; Vigil, M.F.; Haley, S.D. Genotypic differences for nitrogen use efficiency and grain protein deviation in hard winter wheat. Agron. J. 2016, 108, 2201–2213. [Google Scholar] [CrossRef]
- Quemada, M.; Lassaletta, L.; Jensen, L.S.; Godinot, O.; Brentrup, F.; Buckley, C.; Foray, S.; Hvid, S.K.; Oenema, J.; Richards, K.G.; et al. Exploring nitrogen indicators of farm performance among farm types across several European case studies. Agric. Syst. 2020, 177, 102689. [Google Scholar] [CrossRef]
- Meisner, C.A.; Acevedo, E.; Flores, D.; Sayre, K.; Ortiz-Monasterio, J.I.; Byerlee, D. Wheat Production and Grower Practices in the Yaqui Valley, Sonora, Mexico; Wheat Special Report No. 6; CIMMYT: Texoco, Mexico, 1992. [Google Scholar]
- American Association of Cereal Chemists (AACC). AACC Standard Methods 39–10 and 46–11A. Approved Methods of Analysis, 11th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2012; ISBN 978-1-891127-68-2. [Google Scholar]
- Gueymard, C.A.; Myers, D.; Emery, K. Proposed reference irradiance spectra for solar energy systems testing. Sol. Energy 2002, 73, 443–467. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakaiya, E.; Zhu, Y.; Takahashi, W. Diagnostic mapping of canopy nitrogen content in rice based on hyperspectral measurements. Remote Sens. Environ. 2012, 126, 210–221. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steve, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–1997. [Google Scholar] [CrossRef]
- Kim, M.; Daughtry, C.; Chapelle, E.; McMurtrey, J.; Walthall, C. The use of high spectral resolution bands for estimating absorbed photosynthetically active radiation (Apar). In 6th Symposium on Physical Measurements and Signatures in Remote Sensing; ISPRS: Val D’ Isere, France, 1994; pp. 299–306. [Google Scholar]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, L08403. [Google Scholar] [CrossRef] [Green Version]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-wave band spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, V.; Berni, J.A.J. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.R.; de Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Fitzgerald, G.J.; Rodriguez, D.; Christensen, L.K.; Belford, R.; Sadras, V.O.; Clarke, T.R. Spectral and thermal sensing for nitrogen and water status in rainfed and irrigated wheat environments. Precis. Agric. 2006, 7, 233–248. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Scaling-up and model inversion methods with narrow-band optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef] [Green Version]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J. Monitoring the Vernal Advancement of Retrogradation of Natural Vegetation; Tipe III (Final Report); NASA/GSFC: Greenbelt, MD, USA, 1974; pp. 1–371. [Google Scholar]
- Roujean, J.L.; Breon, F.M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Chen, P.; Haboudane, D.; Tremblay, N.; Wang, J.; Vigneault, P.; Li, B. New spectral indicator assessing the efficiency of crop nitrogen treatment in corn and wheat. Remote Sens. Environ. 2010, 114, 1987–1997. [Google Scholar] [CrossRef]
- Inoue, Y.; Guérif, M.; Baret, F.; Skidmore, A.; Gitelson, A.; Schlerf, M.; Darvishzadeh, R.; Olioso, A. Simple and robust methods for remote sensing of canopy chlorophyll content: A comparative analysis of hyperspectral data for different types of vegetation. Plant. Cell Environ. 2016, 39, 2609–2623. [Google Scholar] [CrossRef] [Green Version]
- Arregui, L.M.; Lasa, B.; Lafarga, A.; Irañeta, I.; Baroja, E.; Quemada, M. Evaluation of chlorophyll meters as tools for N fertilization in winter wheat under humid Mediterranean conditions. Eur. J. Agron. 2006, 24, 140–148. [Google Scholar] [CrossRef]
- Quemada, M.; Gabriel, J.L.; Zarco-Tejada, P. Airborne hyperspectral images and ground-level optical sensors as assessment tools for maize nitrogen fertilization. Remote Sens. 2014, 6, 2940–2962. [Google Scholar] [CrossRef] [Green Version]
- Varvel, G.E.; Schepers, J.S.; Francis, D.D. Ability for in season correction of nitrogen deficiency in corn using chlorophyll meter. Soil Sci. Soc. Am. J. 1997, 61, 1233–1239. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: http://www.r-project.org/ (accessed on 30 April 2020).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. lme4: Linear Mixed-Effects Using “Eigen” and S4. R Package Version 1.1-23. 2015. Available online: https://CRAN.R-project.org/package=lme4 (accessed on 30 April 2020).
- Babar, M.A.; Reynolds, M.P.; Van Ginkel, M.; Klatt, A.R.; Raun, W.R.; Stone, M.L. Spectral reflectance to estimate genetic variation for in?season biomass, leaf chlorophyll, and canopy temperature in wheat. Crop Sci. 2006, 46, 1046–1057. [Google Scholar] [CrossRef]
- Babar, M.A.; Reynolds, M.P.; Van Ginkel, M.; Klatt, A.R.; Raun, W.R.; Stone, M.L. Spectral reflectance indices as a potential indirect selection criteria for wheat yield under irrigation. Crop Sci. 2006, 46, 578–588. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Hennig, S.D.; Gnyp, M.L.; Chen, X.; Jia, L.; Baret, G. Evaluating hyperspectral vegetation indices for estimating nitrogen concentration of winter wheat at different growth stages. Precis. Agric. 2010, 11, 335–357. [Google Scholar] [CrossRef]
- Prasad, B.; Carver, B.F.; Stone, M.L.; Babar, M.A.; Raun, W.R.; Klatt, A.R. Potential use of spectral reflectance indices as a selection tool for grain yield in winter wheat under great plains conditions. Crop Sci. 2007, 47, 1426–1440. [Google Scholar] [CrossRef] [Green Version]
- Eitel, J.U.H.; Long, D.S.; Gessler, P.E.; Hunt, E.R. Combined spectral index to improve ground-based estimates of nitrogen status in dryland wheat. Agron. J. 2008, 100, 1694–1702. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Mistele, B.; Hu, Y.; Chen, X.; Schmidhalter, U. Comparing hyperspectral index optimization algorithms to estimate aerial N uptake using multi-temporal winter wheat datasets from contrasting climatic and geographic zones in China and Germany. Agric. For. Meteorol. 2013, 180, 44–57. [Google Scholar] [CrossRef]
- Cammarano, D.; Fitzgerald, G.J.; Casa, R.; Basso, B. Assessing the robustness of vegetation indices to estimate wheat N in mediterranean environments. Remote Sens. 2014, 6, 2827–2844. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.A.; Ramos, O.M.; Monasterio, I.O.; Sayre, K.D. Yield response to plant density, row spacing and raised beds in low latitude spring wheat with ample soil resources: An update. Field Crops Res. 2019, 232, 95–105. [Google Scholar] [CrossRef]
- EUNEP. Nitrogen Use Efficiency (NUE) an Indicator for the Utilization of Nitrogen in Food Systems; Wageningen University, Alterra: Wageningen, The Netherlands, 2015. [Google Scholar]
- Gutierrez, M.; Reynolds, M.P.; Klatt, A.R. Association of water spectral indices with plant and soil water relations in contrasting wheat genotypes. J. Exp. Bot. 2010, 61, 3291–3303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altenbach, S.B.; DuPont, F.M.; Kothari, K.M.; Chan, R.; Johnson, E.L.; Lieu, D. Temperature, water and fertilizer influence the timing of key events during grain development in a US spring wheat. J. Cereal Sci. 2003, 37, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Triboï, E.; Martre, P.; Triboï Blondel, A.M. Environmentally-induced changes in protein composition in developing grains of wheat are related to changes in total protein content. J. Exp. Bot. 2003, 54, 1731–1742. [Google Scholar] [CrossRef]
- Gooding, M.J.; Ellis, R.H.; Shewry, P.R.; Schofield, J.D. Effects of restricted water availability and increased temperature on the grain filling, drying and quality of winter wheat. J. Cereal Sci. 2003, 37, 295–309. [Google Scholar] [CrossRef]
- Quemada, M.; Gabriel, J.L. Approaches for increasing nitrogen and water use efficiency simultaneously. Glob. Food Sec. 2016, 9, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Whelan, B.M.; Taylor, J.A.; Hassall, J.A. Site-specific variation in wheat grain protein concentration and wheat grain yield measured on an Australian farm using harvester-mounted on-the-go sensors. Crop Pasture Sci. 2009, 60, 808–817. [Google Scholar] [CrossRef]
- Mulla, D. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Franzen, D.W.; Kitchen, N.R.; Holland, K.H.; Schepers, J.S.; Raun, W.R. Algorithms for in-season nutrient management in cereals. Agron. J. 2016, 108, 1775. [Google Scholar] [CrossRef] [Green Version]
- Blankenau, K.; Olfs, H.W.; Kuhlmann, H. Strategies to improve the use efficiency of mineral fertilizer nitrogen applied to winter wheat. J. Agron. Crop Sci. 2002, 188, 146–154. [Google Scholar] [CrossRef]
- Bogard, M.; Allard, V.; Brancourt-Hulmel, M.; Heumez, E.; MacHet, J.M.; Jeuffroy, M.H.; Gate, P.; Martre, P.; Le Gouis, J. Deviation from the grain protein concentration-grain yield negative relationship is highly correlated to post-anthesis N uptake in winter wheat. J. Exp. Bot. 2010, 61, 4303–4312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebourgeois, V.; Dupuy, S.; Vintrou, É.; Ameline, M.; Butler, S.; Bégué, A. A combined random forest and OBIA classification scheme for mapping smallholder agriculture at different nomenclature levels using multisource data (simulated Sentinel-2 time series, VHRS and DEM). Remote Sens. 2017, 9, 259. [Google Scholar] [CrossRef] [Green Version]
- Mutanga, O.; Adam, E.; Cho, M.A. High density biomass estimation for wetland vegetation using WorldView-2 imagery and random forest regression algorithm. Int. J. Appl. Earth Obs. 2012, 18, 399–406. [Google Scholar] [CrossRef]










| Index | Equation | Ref. |
|---|---|---|
| Greenness or structural indices | ||
| Normalized difference vegetation index | NDVI = (R800 − R670)/(R800 + R670) | [54] |
| Reformed difference vegetation index | RDVI = (R800 − R670)/(R800 + R670)0.5 | [55] |
| Normalized green | NG = R800/R550 | [52] |
| Chlorophyll indices | ||
| Red-edge optical reflectance | R750/R710 | [53] |
| Double peak canopy nitrogen index | DCNI = (R720 − R700)/(R700 − R670)/(R720 − R670 + 0.03) | [56] |
| Modified chlorophyll absorption ratio index | MCARI = [(R700 − R670) − 0.2(R700 − R550)] (R700/R670) | [52] |
| Normalized difference red-edge | NDRE = (R790 − R720)/(R790 + R720) | [51] |
| Canopy chlorophyll content | ||
| Canopy chlorophyll content | CCC = 0.325 (R815/R704) − 0.358 | [57] |
| Canopy chlorophyll content index | CCCI = (NDRE − NDREmin)/(NDREmax − NDREmin) | [51] |
| Nitrogen planar domain index | NDPI = (CIred-edge − CIred-edge min)/(CIred-edge max − CIred-edge min) | [20] |
| R2 | RMSE (kg ha−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indices | Grain Yield | Biomass | Grain Yield | Biomass | ||||||||
| GS31 | GS65 | GS73 | GS31 | GS65 | GS73 | GS31 | GS65 | GS73 | GS31 | GS65 | GS73 | |
| NDVI | 0.60 | 0.72 | 0.65 | 0.46 | 0.72 | 0.62 | 771 | 596 | 678 | 1552 | 988 | 1226 |
| RDVI | 0.65 | 0.76 | 0.68 | 0.51 | 0.76 | 0.66 | 727 | 556 | 648 | 1469 | 906 | 1161 |
| NG | 0.44 | 0.42 | 0.29 | 0.27 | 0.40 | 0.27 | 909 | 868 | 956 | 1796 | 1440 | 1697 |
| NDRE | 0.51 | 0.72 | 0.61 | 0.43 | 0.71 | 0.59 | 852 | 602 | 714 | 1596 | 998 | 1275 |
| R750/R710 | 0.50 | 0.68 | 0.58 | 0.39 | 0.70 | 0.57 | 873 | 643 | 735 | 1648 | 1029 | 1297 |
| DCNI | 0 | 0 | 0.12 | 0.01 | 0 | 0.13 | 1220 | 1136 | 1070 | 2098 | 1865 | 1850 |
| MCARI | 0.09 | 0.26 | 0.27 | 0.05 | 0.27 | 0.25 | 1163 | 981 | 972 | 2053 | 1594 | 1712 |
| CCC | 0.49 | 0.66 | 0.62 | 0.37 | 0.68 | 0.61 | 871 | 664 | 704 | 1671 | 1063 | 1232 |
| CCCI | 0.19 | 0.21 | 0.27 | 0.20 | 0.20 | 0.26 | 1099 | 1013 | 973 | 1878 | 1666 | 1698 |
| NDPI | 0.22 | 0.52 | 0.46 | 0.19 | 0.52 | 0.44 | 1072 | 784 | 840 | 1797 | 1295 | 1475 |
| Indices | R2 | RMSE (%N) | ||||||
|---|---|---|---|---|---|---|---|---|
| GS41 | GS51 | GS65 | GS73 | GS41 | GS51 | GS65 | GS73 | |
| Year 2014 | ||||||||
| NDVI | 0.13 | 0.39 | 0.35 | 0.65 | 0.31 | 0.26 | 0.27 | 0.20 |
| RDVI | 0.11 | 0.27 | 0.37 | 0.59 | 0.32 | 0.29 | 0.27 | 0.21 |
| NG | 0.09 | 0.26 | 0.30 | 0.64 | 0.32 | 0.29 | 0.28 | 0.20 |
| NDRE | 0.20 | 0.28 | 0.39 | 0.69 | 0.30 | 0.28 | 0.26 | 0.19 |
| R750/R710 | 0.12 | 0.26 | 0.33 | 0.65 | 0.32 | 0.29 | 0.28 | 0.20 |
| DCNI | 0.61 | 0.01 | 0.30 | 0.78 | 0.21 | 0.33 | 0.28 | 0.16 |
| MCARI | 0.00 | 0.40 | 0.06 | 0.34 | 0.33 | 0.26 | 0.33 | 0.27 |
| CCC | 0.11 | 0.21 | 0.27 | 0.53 | 0.32 | 0.30 | 0.29 | 0.23 |
| CCCI | 0.47 | 0.09 | 0.54 | 0.58 | 0.25 | 0.32 | 0.23 | 0.22 |
| NDPI | 0.24 | 0.17 | 0.39 | 0.65 | 0.29 | 0.30 | 0.26 | 0.20 |
| Year 2015 | ||||||||
| NDVI | 0.22 | 0.41 | 0.44 | 0.50 | 0.19 | 0.20 | 0.12 | 0.12 |
| RDVI | 0.17 | 0.43 | 0.46 | 0.49 | 0.20 | 0.19 | 0.12 | 0.12 |
| NG | 0.17 | 0.42 | 0.29 | 0.38 | 0.20 | 0.20 | 0.14 | 0.13 |
| NDRE | 0.23 | 0.42 | 0.36 | 0.49 | 0.19 | 0.20 | 0.13 | 0.12 |
| R750/R710 | 0.18 | 0.42 | 0.31 | 0.42 | 0.20 | 0.20 | 0.14 | 0.13 |
| DCNI | 0.08 | 0.07 | 0.06 | 0.52 | 0.21 | 0.25 | 0.16 | 0.11 |
| MCARI | 0.02 | 0.33 | 0.49 | 0.28 | 0.22 | 0.21 | 0.12 | 0.14 |
| CCC | 0.18 | 0.42 | 0.31 | 0.41 | 0.20 | 0.20 | 0.14 | 0.13 |
| CCCI | 0.18 | 0.24 | 0.22 | 0.48 | 0.20 | 0.22 | 0.15 | 0.12 |
| NDPI | 0.00 | 0.34 | 0.24 | 0.43 | 0.22 | 0.21 | 0.14 | 0.12 |
| Year 2016 | ||||||||
| NDVI | 0.38 | 0.83 | 0.26 | 0.89 | 0.22 | 0.14 | 0.27 | 0.11 |
| RDVI | 0.28 | 0.84 | 0.24 | 0.90 | 0.24 | 0.14 | 0.28 | 0.11 |
| NG | 0.39 | 0.85 | 0.25 | 0.90 | 0.22 | 0.13 | 0.27 | 0.11 |
| NDRE | 0.31 | 0.84 | 0.36 | 0.92 | 0.23 | 0.14 | 0.25 | 0.10 |
| R750/R710 | 0.32 | 0.87 | 0.30 | 0.91 | 0.23 | 0.12 | 0.27 | 0.10 |
| DCNI | 0.00 | 0.04 | 0.09 | 0.18 | 0.28 | 0.34 | 0.30 | 0.31 |
| MCARI | 0.29 | 0.63 | 0.00 | 0.58 | 0.23 | 0.21 | 0.32 | 0.22 |
| CCC | 0.31 | 0.87 | 0.28 | 0.91 | 0.23 | 0.13 | 0.27 | 0.10 |
| CCCI | 0.13 | 0.75 | 0.36 | 0.88 | 0.26 | 0.17 | 0.25 | 0.12 |
| NDPI | 0.23 | 0.85 | 0.37 | 0.93 | 0.24 | 0.13 | 0.25 | 0.09 |
| Year 2017 | ||||||||
| NDVI | 0.03 | 0.55 | 0.56 | 0.69 | 0.35 | 0.24 | 0.23 | 0.19 |
| RDVI | 0.01 | 0.53 | 0.53 | 0.73 | 0.35 | 0.24 | 0.23 | 0.18 |
| NG | 0.01 | 0.68 | 0.53 | 0.70 | 0.35 | 0.20 | 0.23 | 0.19 |
| NDRE | 0.07 | 0.56 | 0.53 | 0.76 | 0.34 | 0.23 | 0.23 | 0.17 |
| R750/R710 | 0.02 | 0.55 | 0.46 | 0.78 | 0.35 | 0.24 | 0.25 | 0.16 |
| DCNI | 0.50 | 0.13 | 0.06 | 0.21 | 0.25 | 0.33 | 0.33 | 0.30 |
| MCARI | 0.07 | 0.28 | 0.44 | 0.48 | 0.34 | 0.30 | 0.25 | 0.25 |
| CCC | 0.02 | 0.45 | 0.42 | 0.72 | 0.35 | 0.26 | 0.26 | 0.18 |
| CCCI | 0.45 | 0.57 | 0.49 | 0.27 | 0.26 | 0.23 | 0.24 | 0.29 |
| NDPI | 0.10 | 0.56 | 0.44 | 0.72 | 0.34 | 0.24 | 0.26 | 0.18 |
| Indices | R2 | RMSE (kg N ha−1) | ||||
|---|---|---|---|---|---|---|
| GS31 | GS65 | GS73 | GS31 | GS65 | GS73 | |
| NDVI | 0.55 | 0.75 | 0.81 | 16 | 11 | 10 |
| RDVI | 0.57 | 0.81 | 0.82 | 16 | 10 | 10 |
| NG | 0.34 | 0.36 | 0.36 | 19 | 18 | 19 |
| NDRE | 0.48 | 0.84 | 0.79 | 17 | 9 | 11 |
| R750/R710 | 0.47 | 0.76 | 0.77 | 17 | 11 | 12 |
| DCNI | 0.01 | 0.02 | 0.13 | 23 | 23 | 22 |
| MCARI | 0.06 | 0.15 | 0.25 | 23 | 21 | 21 |
| CCC | 0.43 | 0.71 | 0.78 | 18 | 12 | 11 |
| CCCI | 0.21 | 0.35 | 0.39 | 21 | 18 | 19 |
| NDPI | 0.20 | 0.68 | 0.45 | 21 | 13 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raya-Sereno, M.D.; Ortiz-Monasterio, J.I.; Alonso-Ayuso, M.; Rodrigues, F.A., Jr.; Rodríguez, A.A.; González-Perez, L.; Quemada, M. High-Resolution Airborne Hyperspectral Imagery for Assessing Yield, Biomass, Grain N Concentration, and N Output in Spring Wheat. Remote Sens. 2021, 13, 1373. https://doi.org/10.3390/rs13071373
Raya-Sereno MD, Ortiz-Monasterio JI, Alonso-Ayuso M, Rodrigues FA Jr., Rodríguez AA, González-Perez L, Quemada M. High-Resolution Airborne Hyperspectral Imagery for Assessing Yield, Biomass, Grain N Concentration, and N Output in Spring Wheat. Remote Sensing. 2021; 13(7):1373. https://doi.org/10.3390/rs13071373
Chicago/Turabian StyleRaya-Sereno, María D., J. Ivan Ortiz-Monasterio, María Alonso-Ayuso, Francelino A. Rodrigues, Jr., Arlet A. Rodríguez, Lorena González-Perez, and Miguel Quemada. 2021. "High-Resolution Airborne Hyperspectral Imagery for Assessing Yield, Biomass, Grain N Concentration, and N Output in Spring Wheat" Remote Sensing 13, no. 7: 1373. https://doi.org/10.3390/rs13071373