- 1Department of Gastroenterology, General Hospital of Northern Theater Command, Shenyang, China
- 2Postgraduate College, China Medical University, Shenyang, China
- 3Postgraduate College, Shenyang Pharmaceutical University, Shenyang, China
- 4Department of Thoracic Surgery, General Hospital of Northern Theater Command, Shenyang, China
- 5Information Section of Medical Security Center, General Hospital of Northern Theater Command, Shenyang, China
Background: Active and severe ulcerative colitis (UC) and non-response to 5-aminosalicylic acid (5-ASA) are related to poor outcomes and should be accurately identified. Several integrated inflammatory indexes are potentially useful to assess the disease severity in patients with acute or critical diseases but are underexplored in patients with UC.
Methods: Patients with UC consecutively admitted to our hospital between January 2015 and December 2020 were retrospectively grouped according to the activity and severity of UC and response to 5-ASA. The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), neutrophil-to-platelet ratio (NPR), platelet-to-albumin ratio (PAR), C-reactive protein-to-albumin ratio (CAR), and C-reactive protein-to-lymphocyte ratio (CLR) were calculated. The areas under receiver operating characteristic curves (AUC) were calculated.
Results: Overall, 187 patients with UC were included, of whom 151 were active, 55 were severe, and 14 were unresponsive to 5-ASA. The active UC group had significantly higher NLR, PLR, SII, and PAR levels. SII had the greatest predictive accuracy for active UC, followed by PLR, PAR, and NLR (AUC = 0.647, 0.641, 0.634, and 0.626). The severe UC group had significantly higher NLR, PLR, SII, PAR, CAR, and CLR levels. CLR had the greatest predictive accuracy for severe UC, followed by CAR, PLR, SII, NLR, and PAR (AUC = 0.732, 0.714, 0.693, 0.669, 0.646, and 0.63). The non-response to the 5-ASA group had significantly higher CAR and CLR levels. CAR had a greater predictive accuracy for non-response to 5-ASA than CLR (AUC = 0.781 and 0.759).
Conclusion: SII, CLR, and CAR may be useful for assessing the severity and progression of UC, but remain not optimal.
Introduction
Ulcerative colitis (UC) is a relapsing and remitting mucosal inflammation often restricted to the colon and rectum, which may be associated with dysregulated immune response (1). The highest incidence and prevalence of UC is 4.6 per 100,000 person-years and 57.3 per 100,000 persons in Eastern Asia, respectively (2). Patients with active UC always suffer from embarrassing and painful symptoms, such as fecal incontinence, abdominal pain, bloody diarrhea, arthritis, and fatigue, and tend to develop poorer psychosocial outcomes than those with inactive UC (3). Active UC is usually classified into mild, moderate, and severe according to the recommendation by the international guideline (4). Generally, severe UC can bring more negative impact on the patient's quality of life, social and psychological wellbeing, healthcare resource utilization (5), and prognosis than mild-moderate UC (6, 7). Early medications can avoid the progression from mild-moderate to severe UC (7). 5-aminosalicylic acid (5-ASA) is the first-line choice of medication for patients with UC diagnosed within the first year (8). Usually, patients with severe UC are not well responsive to 5-ASA, leading to the use of corticosteroids, immunosuppressants, and biologics (9–12). Therefore, it is a clinical priority to identify patients who require more aggressive treatment to reach clinical remission.
In 1955, Truelove and Witts (13) established their criteria to explore the efficacy of cortisone medications in patients with UC. Currently, the Truelove and Witts criteria have been the cornerstone of assessing the severity of UC. However, it still has several potential limitations. The most critical limitation is that the definitions of improvement and worsening are ambiguous (14), as well as that of moderate UC. In 1987, Schroeder et al. (15) further developed the Mayo score by combining clinical symptoms, laboratory tests, and endoscopic findings. Despite one of the most commonly used scores for the severity of UC, it contains endoscopic procedures, which may be invasive, expensive, and time-consuming. Recently, the partial Mayo score (PMS) has been increasingly recognized, because it can properly discriminate this disease based on stool frequency, rectal bleeding, and physician's global assessment, but does not contemplate endoscopic data (16–18). However, the requirement of clinician's subjective evaluation of patient's symptoms remains its potential drawback (19).
Erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) are two traditional serologic biomarkers and are usually used for monitoring the disease course of UC, but their sensitivity and specificity are unsatisfactory (20). Recently, several integrated indexes have been used for the assessment of infective, acute, and critical diseases, including neutrophil-to-lymphocyte ratio (NLR) (21), platelet-to-lymphocyte ratio (PLR) (22), systemic immune-inflammation index (SII) (23), neutrophil-to-platelet ratio (NPR) (24), platelet-to-albumin ratio (PAR) (25), CRP-to-albumin ratio (CAR) (26), and CRP-to-lymphocyte ratio (CLR) (27). Notably, they are non-invasive and easier to use at a low cost. Thus, the purpose of this study is to determine the accuracy of inflammatory indexes for diagnosing active UC and severe UC and identifying the response to 5-ASA medication.
Methods
Patient Selection
This is a single-center retrospective, cross-sectional study. We extracted the medical records of all UC patients who were consecutively admitted to the General Hospital of Northern Theater Command between January 1, 2015 and December 31, 2020 from the inpatient information system. The exclusion criteria were as follows: (1) medical records cannot be reviewed in detail; (2) patients were diagnosed as suspected UC and unclassified inflammatory bowel disease; (3) routine blood tests were missing; (4) history of colectomy; (5) co-existing conditions that potentially influence inflammatory indexes (i.e., severe trauma, pregnancy, liver cirrhosis, uremia, and malignancy); and (6) other autoimmune diseases (i.e., psoriasis, Behcet's disease, urticarial vasculitis, and rheumatoid arthritis). The study protocol was approved by the Ethical Committee of General Hospital of Northern Theater Command [Y (2021) 078] and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The requirement of informed written consent was waived because only the data from the inpatient's electronic medical records were extracted.
Data Collection
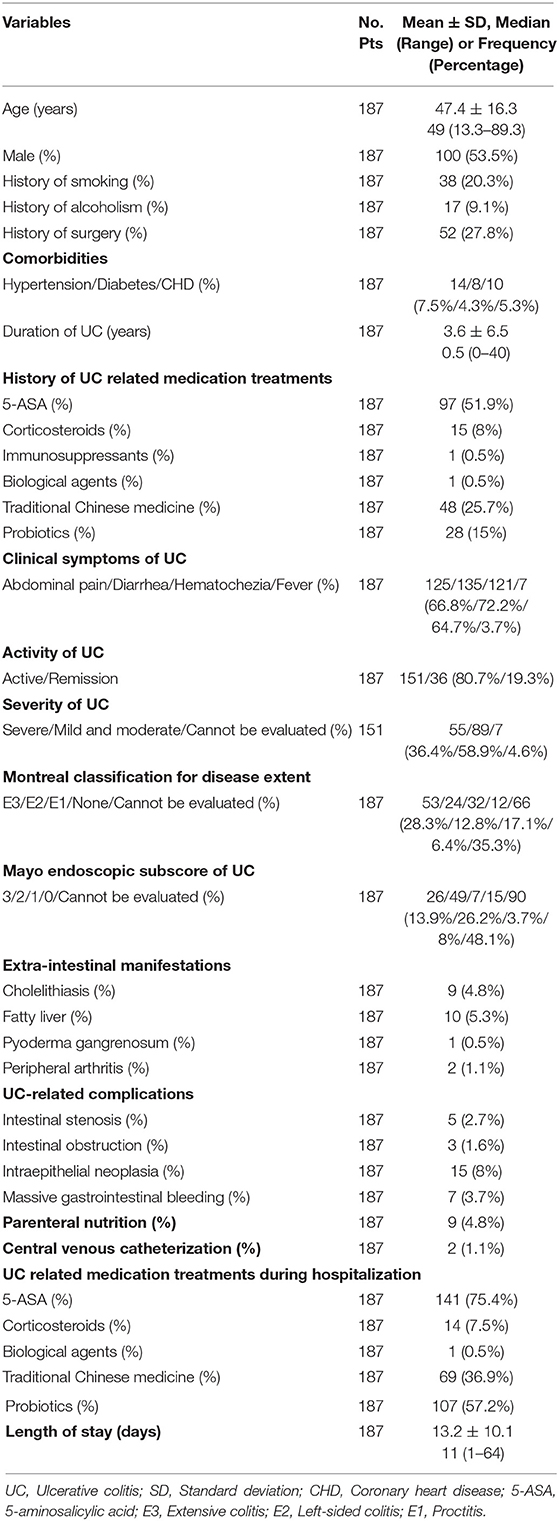
The patient's demographics, history of surgery, comorbidities, duration of UC, history of UC-related medication treatments, clinical symptoms of UC (i.e., abdominal pain, diarrhea, hematochezia, and fever), endoscopic reports, blood tests at admission, extra-intestinal manifestations, UC-related complications, UC-related medications during hospitalization, and length of stay (LOS) were manually extracted from the inpatient's electronic medical records. Fever was defined, if the body temperature, which was measured on the first day of admission according to the electronic medical records, was > 37.3°C. The Montreal classification for disease extent of UC (28) and Mayo endoscopic subscore (29) were also assessed through endoscopic reports. Several inflammatory indexes, including NLR, PLR, SII, NPR, PAR, CAR, and CLR, were calculated. NLR was calculated as the neutrophil counts (109/L) divided by the lymphocyte counts (109/L) (21). PLR was calculated as the platelet counts (109/L) divided by the lymphocyte counts (109/L) (22). SII was calculated as the neutrophil counts (109/L) multiplied by the platelet counts (109/L) and divided by the lymphocyte counts (109/L) (23). NPR was calculated as the neutrophil counts (109/L) multiplied by 1,000 and divided by the platelet counts (109/L) (24). PAR was calculated as the platelet counts (109/L) divided by the albumin levels (g/L) (25). CAR was calculated as the CRP levels (mg/L) divided by the albumin levels (g/L) (26). CLR was calculated as the CRP levels (mg/L) divided by the lymphocyte counts (109/L) (27).
Groups
The patients were grouped according to the activity of UC, the severity of active UC, and response to 5-ASA. First, patients were classified into active and remission UC groups according to the modified Mayo score, which has different definitions of clinical activation and remission of UC as compared to the original Mayo score (15, 29). Briefly, the modified Mayo score is calculated based on the stool frequency, rectal bleeding, endoscopic findings, and physician's global assessment. Clinical activation was defined as a total modified Mayo score of ≥3 points. Clinical remission was defined as a total modified Mayo score of ≤2 points without a sub-score of >1 point. Second, patients with active UC were classified into severe and mild-moderate UC groups according to the modified Truelove and Witts criteria (13). Severe UC was defined as a bloody stool frequency ≥6 per day along with at least one sign of systemic toxicity, including pulse rate >90 bpm, temperature > 37.8°C, hemoglobin level < 10.5 g/dl, ESR > 30 mm/h, and/or CRP > 30 mg/l. Third, patients with active UC receiving 5-ASA during their hospitalizations were classified into response and non-response to 5-ASA groups. Response to 5-ASA was defined as corticosteroid-free clinical remission, considering that corticosteroids are alternatives in our patients who did not achieve clinical remission after 5-ASA.
Statistical Analyses
All statistical analyses were conducted using the SPSS 20.0 (SPSS Inc., Chicago, IL, United States of America), the MedCalc 20.0 (MedCalc Software bvba, Ostend, Belgium), and the GraphPad Prism 8.0.2 (GraphPad Software Inc., San Diego, CA, United States of America). Categorical data were summarized as the frequency with percentage. Differences between groups were assessed using the chi-squared test. Continuous data were summarized as mean ± SD and median with range. Differences between groups were assessed using the non-parametric Mann–Whitney U test. Spearman's correlation coefficients were used to analyze the correlation of inflammatory indexes with the activity of UC, the severity of UC, and response to 5-ASA. Correlation coefficients (r) were reported as follows: 0 < r < 1, positive correlation; −1 < r < 0, negative correlation; and r = 0, no correlation. The diagnostic accuracy of the inflammatory indexes for active UC, severe UC, and non-response to 5-ASA medications was identified by receiver operating characteristic (ROC) curves (30). Their optimal cut-off values, area under the curve (AUC) with 95% CI, sensitivity, and specificity were calculated. The optimal cutoff value was determined in the case that the summation of sensitivity and specificity values was the highest, maximizing Youden's index (31). A two-tailed P < 0.05 was considered statistically significant.
Results
Characteristics of Patients
A total of 246 patients with UC were initially identified and reviewed for potential inclusion. Finally, 187 patients were eligible for the final analysis in the study (Figure 1). The mean age was 47.4 ± 16.3 years. The percentage of males was 53.5%. The mean disease duration was 3.6 ± 6.5 years. Nearly half of the eligible patients (51.9%) had already received 5-ASA medications for UC before admission. Diarrhea (72.2%), abdominal pain (66.8%), and hematochezia (64.7%) were the most common clinical symptoms at admission. Extensive colitis was observed in 53 (28.3%) patients through endoscopy. The Mayo endoscopic sub-score of 3 points was observed in 26 (13.9%) patients. One hundred and forty-one (75.4%) patients received 5-ASA medications for UC at our hospitals. The mean length of hospital stay was 13.2 ± 10.1 days (Table 1).
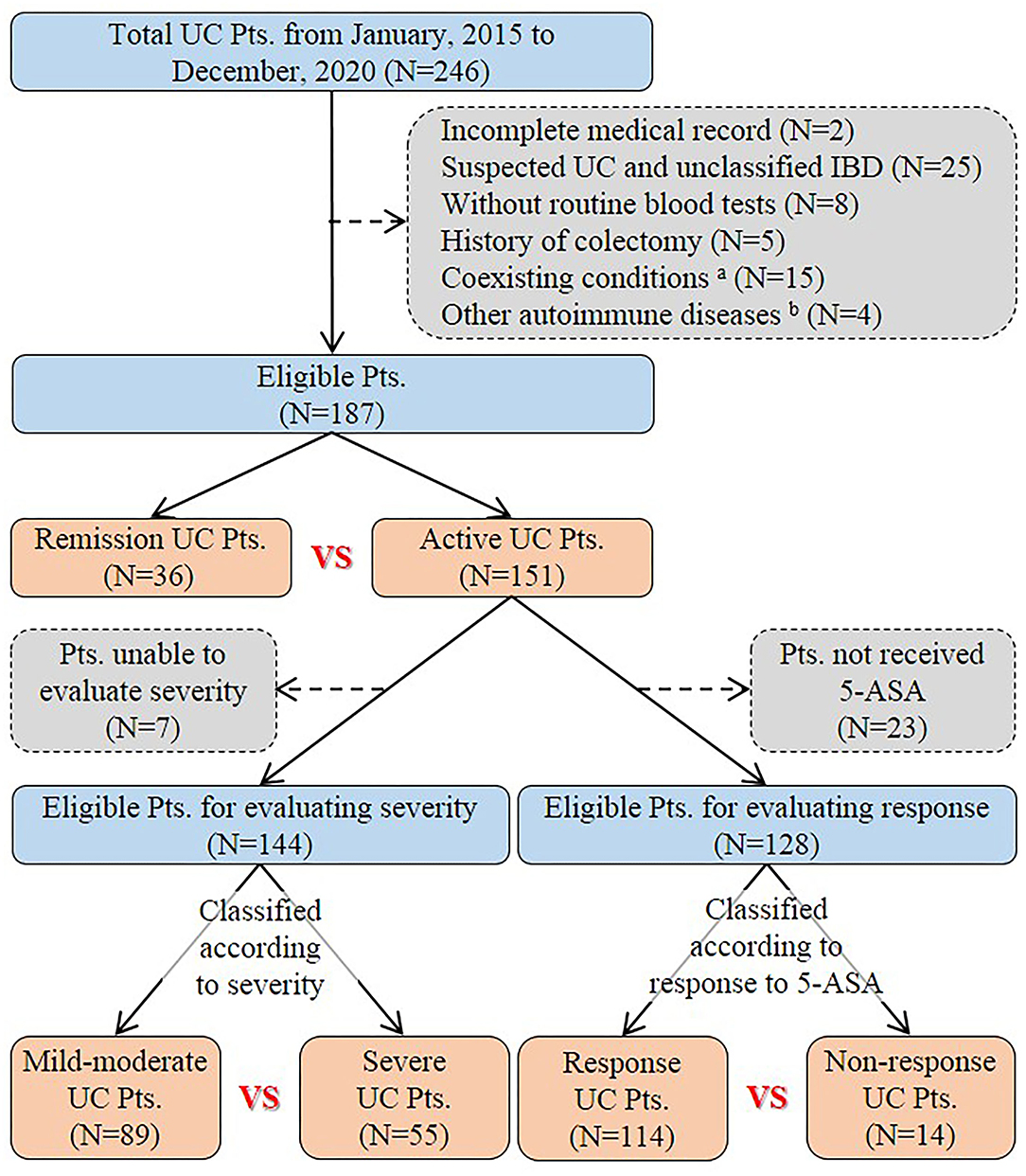
Figure 1. Flowchart of patient selection. aPatients had coexisting conditions that potentially influence inflammatory indexes, including severe trauma (n = 1), pregnancy (n = 1), liver cirrhosis (n = 2), uremia (n = 1), and malignancy (n = 10). bFour patients had other autoimmune diseases, including psoriasis (n = 1), Behcet's disease (n = 1), urticarial vasculitis (n = 1), and rheumatoid arthritis (n = 1). UC, Ulcerative colitis; Pts, Patients; IBD, Inflammatory bowel disease; 5-ASA, 5-aminosalicylic acid.
Inflammatory Indexes and Activity of UC
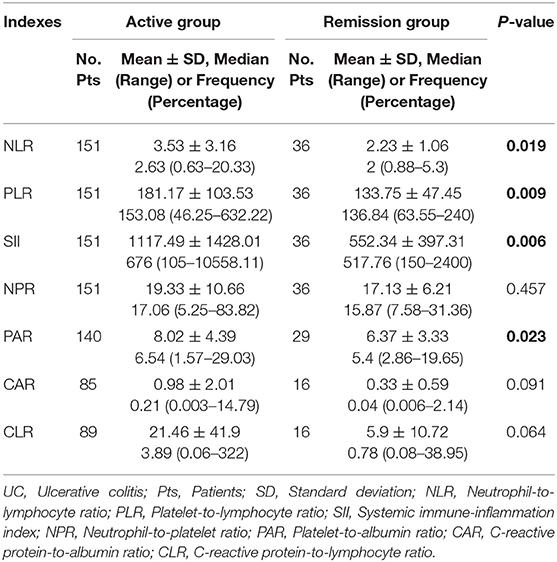
Of the 187 included patients, 151 and 36 were diagnosed with active UC and remission UC, respectively. The mean NLR, PLR, SII, and PAR (P = 0.019, 0.009, 0.006, and 0.023), but not NPR, CAR, or CLR (P = 0.457, 0.091, or 0.064), were significantly higher in the active group than in the remission group (Table 2). NLR, PLR, SII, and PAR were significantly correlated with the activity of UC (P = 0.02, 0.01, 0.01, and 0.02) (Table 3). SII had the largest AUC (AUC = 0.647), followed by CLR, PLR, PAR, CAR, NLR, and NPR (AUC = 0.646, 0.641, 0.634, 0.634, 0.626, and 0.54) (Figure 2). The optimal cut-off value of SII for active UC was 595.47 × 109/L with a sensitivity and specificity of 58.28 and 75%, respectively (Supplementary Table 1). The AUC of SII was significantly different from that of NPR (P = 0.0428), but not NLR, PLR, PAR, CAR, or CLR (P = 0.3506, 0.8508, 0.8233, 0.5929, or 0.6023).
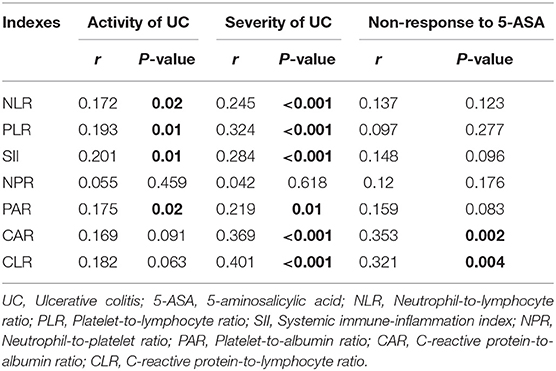
Table 3. Correlation analyses of inflammatory indexes with the activity of UC, the severity of UC, and non-response to 5-aminosalicylic acid (5-ASA).
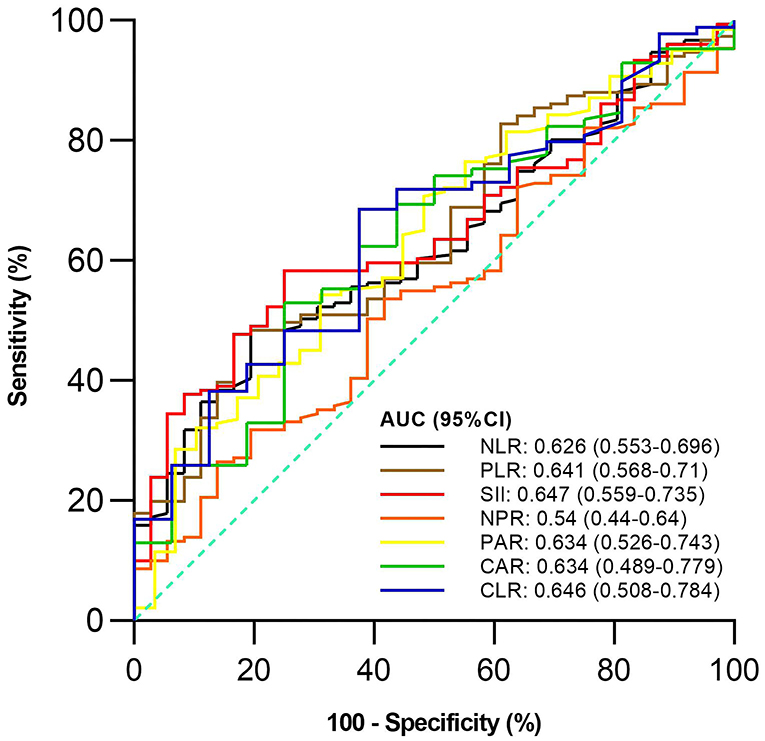
Figure 2. Comparison of predictive performance of inflammatory indexes for active ulcerative colitis (UC). UC, Ulcerative colitis; AUC, Area under the curve; CI, Confidence interval; NLR, Neutrophil-to-lymphocyte ratio; PLR, Platelet-to-lymphocyte ratio; SII, Systemic immune-inflammation index; NPR, Neutrophil-to-platelet ratio; PAR, Platelet-to-albumin ratio; CAR, C-reactive protein-to-albumin ratio; CLR, C-reactive protein-to-lymphocyte ratio.
Inflammatory Indexes and Severity of UC
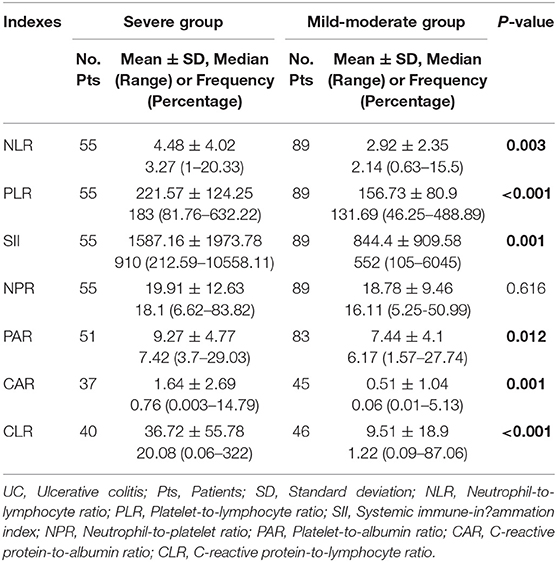
Of the 144 active UC patients, 55 and 89 were diagnosed with severe UC and mild-moderate UC, respectively. The mean NLR, PLR, SII, PAR, CAR, and CLR (P = 0.003, <0.001, 0.001, 0.012, 0.001, and <0.001), but not NPR (P = 0.616), were significantly higher in the severe group than in the mild-moderate group (Table 4). NLR, PLR, SII, PAR, CAR, and CLR were significantly correlated with the severity of UC (P =< 0.001, <0.001, <0.001, 0.01, <0.001, and <0.001) (Table 3). CLR had the largest AUC (AUC = 0.732), followed by CAR, PLR, SII, NLR, PAR, and NPR (AUC = 0.714, 0.693, 0.669, 0.646, 0.63, and 0.525) (Figure 3). The optimal cut-off value of CLR for severe UC was 7 mg/109 with a sensitivity and specificity of 65% and 73.91%, respectively (Supplementary Table 2). The AUC of CLR was significantly different from that of SII, NPR, and PAR (P = 0.0473, 0.043, and 0.0138), but not NLR, PLR, or CAR (P = 0.0562, 0.0723, or 0.1886).
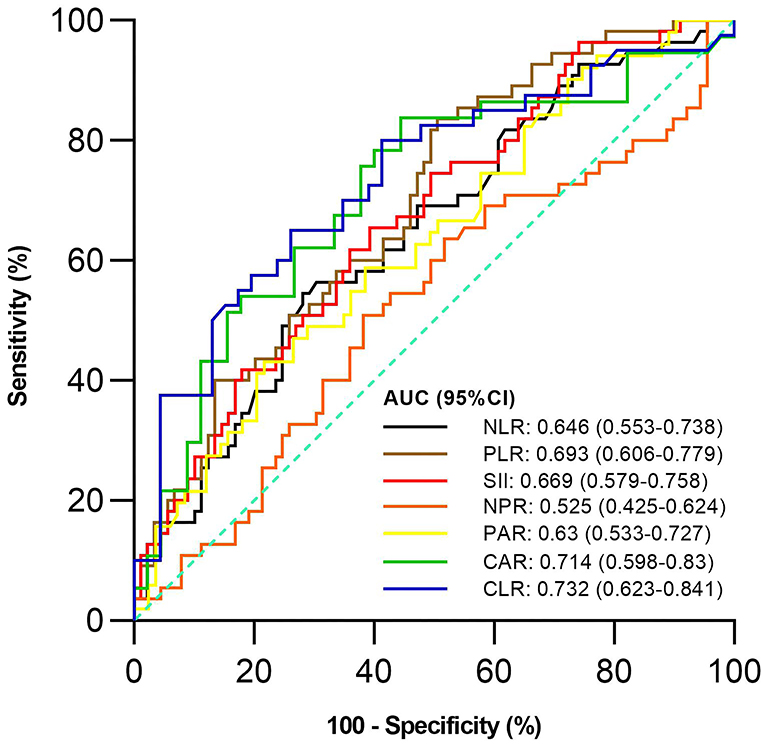
Figure 3. Comparison of the predictive performance of inflammatory indexes for severe UC. UC, Ulcerative colitis; AUC, Area under the curve; CI, Confidence interval; NLR, Neutrophil-to-lymphocyte ratio; PLR, Platelet-to-lymphocyte ratio; SII, Systemic immune-inflammation index; NPR, Neutrophil-to-platelet ratio; PAR, Platelet-to-albumin ratio; CAR, C-reactive protein-to-albumin ratio; CLR, C-reactive protein-to-lymphocyte ratio.
Inflammatory Indexes and Non-Response to 5-ASA
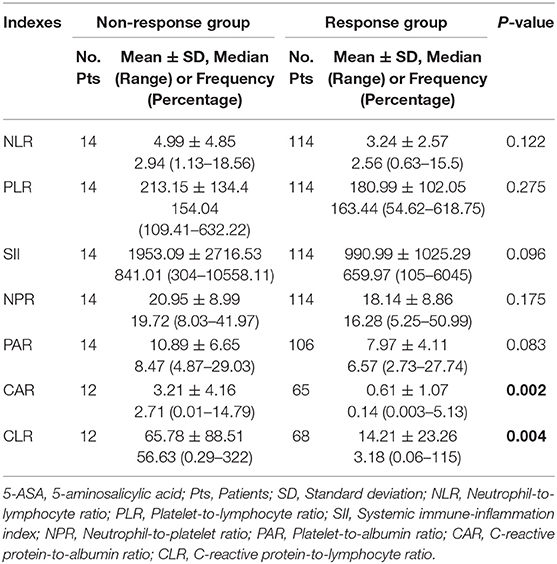
Of the 128 active UC patients who received 5-ASA, 14 were not responsive to 5-ASA. The mean CAR and CLR (P = 0.002 and 0.004), but not NLR, PLR, SII, NPR, and PAR (P = 0.122, 0.275, 0.096, 0.175, and 0.083), were significantly higher in the non-response group than in the response group (Table 5). CAR and CLR were significantly correlated with non-response to 5-ASA (P = 0.002 and 0.004) (Table 3). CAR had the largest AUC (AUC = 0.781), followed by CLR, PAR, SII, NLR, NPR, and PLR (AUC = 0.759, 0.643, 0.637, 0.627, 0.611, and 0.59) (Figure 4). The optimal cut-off value of CAR for non-response to 5-ASA was 2.41mg/g with a sensitivity and specificity of 58.33% and 95.38%, respectively (Supplementary Table 3). The AUC of CAR was significantly different from that of PLR (P = 0.0014), but not NLR, SII, NPR, PAR, or CLR (P = 0.055, 0.0673, 0.1451, 0.1529, or 0.3271).
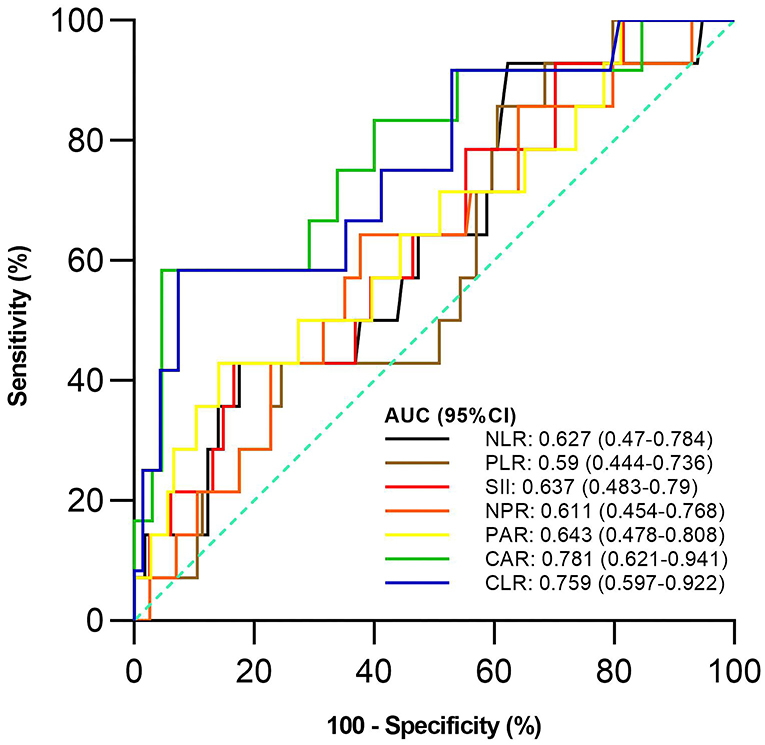
Figure 4. Comparison of the predictive performance of inflammatory indexes for non-response to 5-aminosalicylic acid (5-ASA). 5-ASA, 5-aminosalicylic acid; AUC, Area under the curve; CI, Confidence interval; NLR, Neutrophil-to-lymphocyte ratio; PLR, Platelet-to-lymphocyte ratio; SII, Systemic immune-inflammation index; NPR, Neutrophil-to-platelet ratio; PAR, Platelet-to-albumin ratio; CAR, C-reactive protein-to-albumin ratio; CLR, C-reactive protein-to-lymphocyte ratio.
Discussion
The present study has compared the value of seven inflammatory indexes, including NLR, PLR, SII, NPR, PAR, CAR, and CLR, at baseline for assessing the activity of UC, the severity of UC, and response to 5-ASA, and found that SII had the greatest predictive value for active UC, CLR for severe UC, and CAR for non-response to 5-ASA.
Systemic immune-inflammation index (SII) was originally developed as an independent predictor of recurrence and survival for patients with hepatocellular carcinoma after surgery (23). Recently, it has also been demonstrated that SII level was higher in patients with UC than in healthy control groups and that SII level was positively associated with disease activity in patients (32) with UC. Similarly, our study found that the SII level was significantly correlated with the activity of UC. Moreover, SII had a better diagnostic capability for active UC than other inflammatory indexes. This finding may be attributed to the fact that immunity and inflammation are crucial for the occurrence of UC (1) and SII is calculated as neutrophil counts multiplied by platelet counts and divided by lymphocyte counts (23).
First, in a healthy human body, although 1-2 × 1011 neutrophils are generated every day in the bone marrow (33), chemokine receptors, including CXCR4 and CXCR2, may maintain a delicate balance of neutrophil counts by mediating the retention of neutrophils in the bone marrow and their mobilization to peripheral blood (34, 35). In patients with active inflammatory bowel disease (IBD), there are significantly increased interleukin-17A (IL-17A) levels in inflamed mucosa that promote the transcription of granulocyte colony-stimulating factor in bone marrow (36), thereby inhibiting and activating the expression of CXCR4 binding ligands and CXCR2 binding ligands, respectively. As a result, increased neutrophils are released from the bone marrow into the peripheral blood (37). Therefore, patients with active IBD often have peripheral neutrophilia (38).
Second, lymphocytes, such as Th1 cells, Th17 cells, and B cells, can produce pro-inflammatory cytokines and activate intestinal proteases leading to mucosal damage (39), and are accumulated in the inflamed lamina propria of IBD (40, 41). In patients with active IBD, lymphocytes are accumulated from the peripheral blood into the inflamed intestine, which eventually results in peripheral lymphopenia (39).
Third, platelets are produced from long cytoplasmic processes fragmentation of megakaryocyte in the extravascular marrow space (42). Patients with IBD have elevated levels of thrombopoietin and IL-6, which are involved in megakaryocytic maturation (43). Moreover, in patients with IBD, the platelets in the peripheral blood are active and can spontaneously aggregate with increased susceptibility to proaggregating agents (44). Thus, patients with active UC often have peripheral thrombocytosis (45). Additionally, platelet counts are more strongly associated with the activity of UC than the severity of UC. Platelet counts in peripheral blood are markedly increased in patients with active UC compared with inactive UC (45), but not correlated with the Ulcerative Colitis Colonoscopic Index of Severity, which has an excellent overall assessment of endoscopic severity (46).
C-reactive protein-to-lymphocyte ratio (CLR) was originally developed as a novel index to predict the major morbidity after esophagogastric cancer resection (27). To our knowledge, only Con et al. explored the role of CLR for assessing the dynamic response to infliximab salvage treatment and predicting the risk of subsequent colectomy in patients with UC (47). Similarly, our study found that CLR had a better capability to predict the severity of UC than other inflammatory indexes. This finding may be attributed to the fact that CLR is calculated as CRP levels divided by lymphocyte counts (27). CRP, the most important acute-phase protein, is produced almost exclusively by hepatocytes in response to stimulation by IL-6, IL-1β, and tumor necrosis factor α. In the presence of an acute-phase inflammation or infection, CRP levels are increased dramatically. Contrarily, CRP levels are quickly decreased when inflammation is effectively treated (48). CRP alone can be used to predict the severity of active UC (49). Additionally, CRP levels are more strongly associated with the severity of UC than the activity of UC (50).
C-reactive protein-to-albumin ratio (CAR) was originally developed to predict the outcome of patients from acute medical ward (26). Gibson et al. (51) detected the predictive value of CAR to steroid response in patients with acute severe UC aiming to select patients who need early rescue treatment (51). Similarly, our study found that CAR had a better capability to predict the response to 5-ASA than other inflammatory indexes. This finding may be attributed to the fact that CAR is calculated as CRP levels divided by albumin levels (26). First, CRP levels are significantly correlated with the proportion of corticosteroid use in patients (52) with Crohn's disease. Corticosteroids, as rescue medications, are usually used in patients who are not well responsive to 5-ASA (9, 10). Second, albumin levels can reflect the nutritional status of patients (53) with UC and are correlated significantly with the clinical severity of UC (54). Moreover, an in vitro study suggested a strong interaction between 5-ASA and human serum albumin (55). In a mouse model of UC, 5-ASA conjugated with human serum albumin was found to show a significant therapeutic effect (56). Thus, albumin levels may influence the therapeutic effect of 5-ASA for UC.
The present study had several limitations. First, it was conducted at a single center with a relatively small sample size of total patients and a very small sample size of patients in the non-response group, probably compromising the statistical analyses presented. Second, the external validity of our findings was lacking. Third, endoscopy was not performed by the same expert at our hospital, so endoscopic assessment might be a bit inconsistent. Fourth, this study was retrospective and cross-sectional, where CRP and albumin levels were not routinely tested in our patients. Moreover, there were some damages in the inpatient information system, leading to the lack of details of two patient's medical records. Fifth, only a few patients were treated with corticosteroids or biologics in our study. Therefore, we cannot evaluate the correlation between inflammatory indexes and response to corticosteroids or biologics.
In conclusion, SII, CLR, and CAR have higher diagnostic performance than other inflammatory indexes for active UC, severe UC, and response to 5-ASA, respectively. Dynamic changes of these inflammatory indexes along with activity and severity of UC should be further explored. Moreover, future studies should also evaluate the association between these inflammatory indexes and mucosal severity of UC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee of General Hospital of Northern Theater Command. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
XQ: conceptualization. HL and XQ: methodology. HL, ZB, and XQ: formal analysis and writing–original draft. HL, ZB, GC, and XQ: data curation. HL, ZB, QW, GC, YZ, XG, and XQ: writing–review and editing. XG and XQ: supervision. All authors have made an intellectual contribution to the manuscript and approved the submission.
Funding
YZ received funding supported by the Natural Science Foundation of Liaoning Province (No. 2019-ZD-1058).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The abstract was published in the 5th Korea Digestive Disease Week (KDDW 2021) Conference as a poster presentation, please see the following link: http://people-x.com/kddw2021/KDDW2021_abstract.pdf.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.851295/full#supplementary-material
References
1. Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Primers. (2020) 6:74. doi: 10.1038/s41572-020-0205-x
2. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2017) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0
3. Mikocka-Walus A, Pittet V, Rossel JB, von Känel R. Symptoms of depression and anxiety are independently associated with clinical recurrence of inflammatory bowel disease. Clin Gastroenterol Hepatol. (2016) 14:829–35.e1. doi: 10.1016/j.cgh.2015.12.045
4. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. (2019) 114:384–413. doi: 10.14309/ajg.0000000000000152
5. Coward S, Clement F, Benchimol EI, Bernstein CN, Avina-Zubieta JA, Bitton A, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. (2019) 156:1345–53.e4. doi: 10.1053/j.gastro.2019.01.002
6. Lynch RW, Lowe D, Protheroe A, Driscoll R, Rhodes JM, Arnott ID. Outcomes of rescue therapy in acute severe ulcerative colitis: data from the United Kingdom inflammatory bowel disease audit. Aliment Pharmacol Ther. (2013) 38:935–45. doi: 10.1111/apt.12473
7. Ko CW, Singh S, Feuerstein JD, Falck-Ytter C, Falck-Ytter Y, Cross RK, et al. Clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology. (2019) 156:748–64. doi: 10.1053/j.gastro.2018.12.009
8. Jeuring SF, Bours PH, Zeegers MP, Ambergen TW, van den Heuvel TR, Romberg-Camps MJ, et al. Disease outcome of ulcerative colitis in an era of changing treatment strategies: results from the dutch population-based IBDSL COhort. J Crohns Colitis. (2015) 9:837–45. doi: 10.1093/ecco-jcc/jjv129
9. Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. part 2: current management. J Crohns Colitis. (2017) 11:769–84. doi: 10.1093/ecco-jcc/jjx009
10. De Cassan C, Fiorino G, Danese S. Second-generation corticosteroids for the treatment of Crohn's disease and ulcerative colitis: more effective and less side effects? Dig Dis. (2012) 30:368–75. doi: 10.1159/000338128
11. Bourrier A, Carrat F, Colombel JF, Bouvier AM, Abitbol V, Marteau P, et al. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Aliment Pharmacol Ther. (2016) 43:252–61. doi: 10.1111/apt.13466
12. D'Haens G. Risks and benefits of biologic therapy for inflammatory bowel diseases. Gut. (2007) 56:725–32. doi: 10.1136/gut.2006.103564
13. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. (1955) 2:1041–8. doi: 10.1136/bmj.2.4947.1041
14. Pabla BS, Schwartz DA. Assessing severity of disease in patients with ulcerative colitis. Gastroenterol Clin North Am. (2020) 49:671–88. doi: 10.1016/j.gtc.2020.08.003
15. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. a randomized study. N Engl J Med. (1987) 317:1625–9. doi: 10.1056/NEJM198712243172603
16. Sandborn WJ, Feagan BG, Hanauer S, Vermeire S, Ghosh S, Liu WJ, et al. Long-term efficacy and safety of ozanimod in moderately to severely active ulcerative colitis: results from the open-label extension of the randomized, phase 2 TOUCHSTONE study. J Crohns Colitis. (2021) 15:1120–9. doi: 10.1093/ecco-jcc/jjab012
17. Panaccione R, Danese S, Sandborn WJ, O'Brien CD, Zhou Y, Zhang H, et al. Ustekinumab is effective and safe for ulcerative colitis through 2 years of maintenance therapy. Aliment Pharmacol Ther. (2020) 52:1658–75. doi: 10.1111/apt.16119
18. Amiot A, Filippi J, Abitbol V, Cadiot G, Laharie D, Serrero M, et al. Effectiveness and safety of ustekinumab induction therapy for 103 patients with ulcerative colitis: a GETAID multicentre real-world cohort study. Aliment Pharmacol Ther. (2020) 51:1039–46. doi: 10.1111/apt.15717
19. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. (2006) 55:426–31. doi: 10.1136/gut.2005.069476
20. Barnes EL, Burakoff R. New biomarkers for diagnosing inflammatory bowel disease and assessing treatment outcomes. Inflamm Bowel Dis. (2016) 22:2956–65. doi: 10.1097/MIB.0000000000000903
21. Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. (2001) 102:5–14.
22. Smith RA, Bosonnet L, Ghaneh P, Sutton R, Evans J, Healey P, et al. The platelet-lymphocyte ratio improves the predictive value of serum CA19-9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery. (2008) 143:658–66. doi: 10.1016/j.surg.2007.12.014
23. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
24. Wei XB, Liu YH, He PC, Yu DQ, Tan N, Zhou YL, et al. The impact of admission neutrophil-to-platelet ratio on in-hospital and long-term mortality in patients with infective endocarditis. Clin Chem Lab Med. (2017) 55:899–906. doi: 10.1515/cclm-2016-0527
25. Shirai Y, Shiba H, Haruki K, Horiuchi T, Saito N, Fujiwara Y, et al. Preoperative platelet-to-albumin ratio predicts prognosis of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Anticancer Res. (2017) 37:787–93. doi: 10.21873/anticanres.11378
26. Fairclough E, Cairns E, Hamilton J, Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med. (2009) 9:30–3. doi: 10.7861/clinmedicine.9-1-30
27. Neary C, McAnena P, McAnena O, Kerin M, Collins C. C-Reactive protein-lymphocyte ratio identifies patients at low risk for major morbidity after oesophagogastric resection for cancer. Dig Surg. (2020) 37:515–23. doi: 10.1159/000510963
28. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. (2006) 55:749–53. doi: 10.1136/gut.2005.082909
29. Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2005) 353:2462–76. doi: 10.1056/NEJMoa050516
30. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45. doi: 10.2307/2531595
31. Youden WJ. Index for rating diagnostic tests. Cancer. (1950) 3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3
32. Xie Y, Zhuang T, Ping Y, Zhang Y, Wang X, Yu P, et al. Elevated systemic immune inflammation index level is associated with disease activity in ulcerative colitis patients. Clin Chim Acta. (2021) 517:122–6. doi: 10.1016/j.cca.2021.02.016
33. Borregaard N. Neutrophils, from marrow to microbes. Immunity. (2010) 33:657–70. doi: 10.1016/j.immuni.2010.11.011
34. Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. (2003) 19:583–93. doi: 10.1016/S1074-7613(03)00263-2
35. Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. (2010) 120:2423–31. doi: 10.1172/JCI41649
36. Su J, Chen T, Ji XY, Liu C, Yadav PK, Wu R, et al. IL-25 downregulates Th1/Th17 immune response in an IL-10-dependent manner in inflammatory bowel disease. Inflamm Bowel Dis. (2013) 19:720–8. doi: 10.1097/MIB.0b013e3182802a76
37. Veenstra M, Ransohoff RM. Chemokine receptor CXCR2: physiology regulator and neuroinflammation controller? J Neuroimmunol. (2012) 246:1–9. doi: 10.1016/j.jneuroim.2012.02.016
38. Saniabadi AR, Hanai H, Takeuchi K, Umemura K, Nakashima M, Adachi T, et al. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. (2003) 7:48–59. doi: 10.1046/j.1526-0968.2003.00012.x
39. Giuffrida P, Corazza GR, Di Sabatino A. Old and new lymphocyte players in inflammatory bowel disease. Dig Dis Sci. (2018) 63:277–88. doi: 10.1007/s10620-017-4892-4
40. Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. (2008) 9:1341–6. doi: 10.1038/ni.1659
41. Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. (2008) 9:1347–55. doi: 10.1038/ni.1677
42. Schneider W, Gattermann N. Megakaryocytes: origin of bleeding and thrombotic disorders. Eur J Clin Invest. (1994) 24:16–20. doi: 10.1111/j.1365-2362.1994.tb02420.x
43. Heits F, Stahl M, Ludwig D, Stange EF, Jelkmann W. Elevated serum thrombopoietin and interleukin-6 concentrations in thrombocytosis associated with inflammatory bowel disease. J Interferon Cytokine Res. (1999) 19:757–60. doi: 10.1089/107999099313604
44. Danese S, Motte Cd CdL, Fiocchi C. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol. (2004) 99:938–45. doi: 10.1111/j.1572-0241.2004.04129.x
45. Kapsoritakis AN, Koukourakis MI, Sfiridaki A, Potamianos SP, Kosmadaki MG, Koutroubakis IE, et al. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol. (2001) 96:776–81. doi: 10.1111/j.1572-0241.2001.03621.x
46. Samuel S, Bruining DH, Loftus EV, Thia KT, Schroeder KW, Tremaine WJ, et al. Validation of the ulcerative colitis colonoscopic index of severity and its correlation with disease activity measures. Clin Gastroenterol Hepatol. (2013) 11:49–54.e1. doi: 10.1016/j.cgh.2012.08.003
47. Con D, Andrew B, Nicolaides S, van Langenberg DR, Vasudevan A. Biomarker dynamics during infliximab salvage for acute severe ulcerative colitis: C-reactive protein (CRP)-lymphocyte ratio and CRP-albumin ratio are useful in predicting colectomy. Intest Res. (2022) 20:101–13. doi: 10.5217/ir.2020.00146
48. Karoui S, Laz S, Serghini M, Bibani N, Boubaker J, Filali A. Correlation of C-reactive protein with clinical and endoscopic activity in patients with ulcerative colitis. Dig Dis Sci. (2011) 56:1801–5. doi: 10.1007/s10620-010-1496-7
49. Rosenberg L, Lawlor GO, Zenlea T, Goldsmith JD, Gifford A, Falchuk KR, et al. Predictors of endoscopic inflammation in patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis. (2013) 19:779–84. doi: 10.1097/MIB.0b013e3182802b0e
50. Sonoyama H, Kawashima K, Ishihara S, Kotani S, Fukuba N, Oka A, et al. Capabilities of fecal calprotectin and blood biomarkers as surrogate endoscopic markers according to ulcerative colitis disease type. J Clin Biochem Nutr. (2019) 64:265–70. doi: 10.3164/jcbn.18-92
51. Gibson DJ, Hartery K, Doherty J, Nolan J, Keegan D, Byrne K, et al. CRP/albumin ratio: an early predictor of steroid responsiveness in acute severe ulcerative colitis. J Clin Gastroenterol. (2018) 52:e48–52. doi: 10.1097/MCG.0000000000000884
52. Kwon JH, Im JP, Ye BD, Cheon JH, Jang HJ, Lee KM, et al. Disease phenotype, activity and clinical course prediction based on c-reactive protein levels at diagnosis in patients with crohn's disease: results from the CONNECT study. Gut Liver. (2016) 10:595–603. doi: 10.5009/gnl15411
53. Chen YH, Wang L, Feng SY, Cai WM, Chen XF, Huang ZM. The relationship between C-Reactive protein/albumin ratio and disease activity in patients with inflammatory bowel disease. Gastroenterol Res Pract. (2020) 2020:3467419. doi: 10.1155/2020/3467419
54. Lok KH, Ng CH, Hung HG Li KF, Li KK, Szeto ML. Correlation of serum biomarkers with clinical severity and mucosal inflammation in Chinese ulcerative colitis patients. J Dig Dis. (2008) 9:219–24. doi: 10.1111/j.1751-2980.2008.00350.x
55. Cui FL, Qin LX, Li F, Luo HX. Synchronous fluorescence determination and molecular modeling of 5-Aminosalicylic acid (5-ASA) interacted with human serum albumin. J Mol Model. (2008) 14:1111–7. doi: 10.1007/s00894-008-0352-6
56. Iwao Y, Tomiguchi I, Domura A, Mantaira Y, Minami A, Suzuki T, et al. Inflamed site-specific drug delivery system based on the interaction of human serum albumin nanoparticles with myeloperoxidase in a murine model of experimental colitis. Eur J Pharm Biopharm. (2018) 125:141–7. doi: 10.1016/j.ejpb.2018.01.016
Keywords: ulcerative colitis, inflammatory indexes, activity, severity, 5-aminosalicylic acid
Citation: Lin H, Bai Z, Wu Q, Chu G, Zhang Y, Guo X and Qi X (2022) Inflammatory Indexes for Assessing the Severity and Disease Progression of Ulcerative Colitis: A Single-Center Retrospective Study. Front. Public Health 10:851295. doi: 10.3389/fpubh.2022.851295
Received: 09 January 2022; Accepted: 07 February 2022;
Published: 10 March 2022.
Edited by:
Antonietta G. Gravina, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Raffaele Pellegrino, University of Campania Luigi Vanvitelli, ItalyMaria Mourão, Instituto Politécnico de Viana do Castelo, Portugal
Copyright © 2022 Lin, Bai, Wu, Chu, Zhang, Guo and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingshun Qi, xingshunqi@126.com; Xiaozhong Guo, guo_xiao_zhong@126.com
†These authors have contributed equally to this work
 Hanyang Lin
Hanyang Lin Zhaohui Bai
Zhaohui Bai Qiong Wu4†
Qiong Wu4† Xingshun Qi
Xingshun Qi