- 1Department of Internal Medicine 5, Hematology/Oncology, Friedrich Alexander University Erlangen–Nuremberg, Erlangen, Germany
- 2Department of Internal Medicine III – Hematology and Internal Oncology, University Hospital of Regensburg, Regensburg, Germany
One of the most promising therapeutic approaches for numerous hematological malignancies represents the allogeneic hematopoietic stem cell transplantation (allo-HSCT). One major complication is the development of the life-threatening graft-vs.-host disease (GvHD) which limits beneficial effects of graft-vs.-leukemia (GvL) responses during allo-HSCT. Strengthening GvL effects without induction of severe GvHD is essential to decrease the relapse rate after allo-HSCT. An interesting player in this context is vitamin D3 since it has modulatory capacity in both preventing GvHD and boosting GvL responses. Current studies claim that vitamin D3 induces an immunosuppressive environment by dendritic cell (DC)-dependent generation of regulatory T cells (Tregs). Since vitamin D3 is known to support the antimicrobial defense by re-establishing the physical barrier as well as releasing defensins and antimicrobial peptides, it might also improve graft-vs.-infection (GvI) effects in patients. Beyond that, alloreactive T cells might be attenuated by vitamin D3-mediated inhibition of proliferation and activation. Despite the inhibitory effects of vitamin D3 on T cells, anti-tumor responses of GvL might be reinforced by vitamin D3-triggered phagocytic activity and antibody-based immunotherapy. Therefore, vitamin D3 treatment does not only lead to a shift from a pro-inflammatory toward a tolerogenic state but also promotes tumoricidal activity of immune cells. In this review we focus on vitamin D3 and its immunomodulatory effects by enhancing anti-tumor activity while alleviating harmful allogeneic responses in order to restore the immune balance.
Introduction
The most promising curative therapeutic strategy for a broad spectrum of hematological malignancies remains the allogeneic hematopoietic stem cell transplantation (allo-HSCT) (1). Its efficacy is mainly mediated by alloreactive donor-derived immune cells eliminating malignant host cells, a process known as graft-vs.-leukemia (GvL) effect (2). However, infused donor cells can also attack healthy host tissues due to histocompatibility mismatches, which leads to graft-vs.-host disease (GvHD). This life-threatening complication limits the beneficial effects mediated by GvL. Restoring the host's immune balance during and after transplantation is one of the major challenging obstacles in clinical research (3). Alleviating GvHD responses while boosting anti-leukemia activities could be the key to successful treatment in allo-HSCT. Since both processes underlie more or less the same T cell activity, it is very demanding to dissect GvHD from GvL effects (4). The current standard treatment consists mainly of corticosteroids and calcineurin inhibitors such as cyclosporine and tacrolimus (5). Since these immunosuppressive drugs attenuate T-cell-mediated inflammation (6) and the allo-stimulatory capacity of DCs (7), they lead to alleviation of GvHD symptoms. However, these immunosuppressive mechanisms might reduce GvL effects as well. Recent studies have established promising strategies for strengthening GvL responses without exacerbating GvHD. Infusion of donor lymphocytes, CAR-T cells and checkpoint inhibitors have gained pivotal interest in clinical studies over the past years (8). However, none of these therapeutic approaches target both GvHD and GvL.
Though vitamin D3 has been discovered as an important regulator of calcium homeostasis in the early Twentieth century, its putative immunoregulatory role remained undiscovered until recently (9). Contrary to initial assumptions that vitamin D3 is mainly produced in kidney and liver, vitamin D3 receptor (VDR) and vitamin D3 metabolizing enzymes are also expressed in various types of immune cells (10–12). The novel role of vitamin D3 in regulating effector functions of human macrophages is closely linked to the expression of the vitamin D-1-hydroxylase CYP27B1. The precursor form of vitamin D3 is produced in the epidermis upon ultraviolet B (UVB) irradiation or obtained from dietary intake (13). Vitamin D3-binding protein (VDBP) binds pre-vitamin D3 and is responsible for its transport into the liver. Upon entering the cell, CYP27B1 catalyzes the conversion of 25-hydroxy-vitamin D3 (25(OH)D3) into its bioactive form 1,25-dihydroxy-vitamin D3 (1,25(OH)2D3, calcitriol) (14). Levels of 1,25(OH)2D3 are regulated by the inactivating 1,25(OH)2D3 24-hydroxylase (CYP24A1). 1,25(OH)2D3 binds intracellularly to VDR and induces as a transcription factor the expression of a broad variety of target genes which contain vitamin D3 response elements (VDRE) within their promoters (15) (Figure 1).
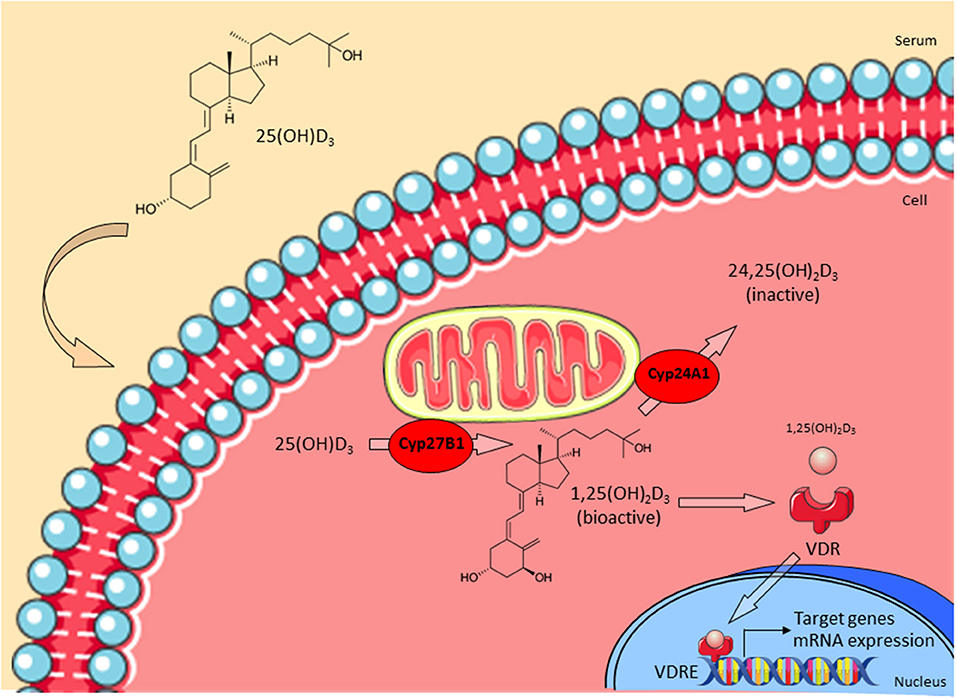
Figure 1. Vitamin D3 metabolism. The precursor form of vitamin D3 25(OH)D3 enters the cell and is then converted into the bioactive form 1,25(OH)2D3 by CYP27B1. CYP24A1 regulates levels of 1,25(OH)2D3 by converting it into the inactive 24,25(OH)2D3. Active 1,25(OH)2D3 binds to the vitamin D3 receptor (VDR) in the cytoplasm and this complex translocates into the nucleus. Finally, VDR binds to appropriate vitamin D response elements (VDRE) and triggers transcription of target genes (e.g., LL-37). Adapted from Bruns and Stenger (14).
Since vitamin D3 is well-known for exerting both anti-tumoricidal and anti-inflammatory functions, it might be an attractive target for preservation of the immune balance in patients undergoing allo-HSCT (16). In this review, we seek to elucidate mechanisms by which vitamin D3 might act as potential immune regulator in GvL as well as GvHD while highlighting its effects on both innate and adaptive immune system.
Vitamin D3 and GvHD
At the beginning of the Twenty-first century, vitamin D3 has gained more attention in the field of allo-HSCT. Given that vitamin D3 exerts “non-classical” actions besides sustaining bone metabolism and calcium homeostasis, paved the way for pioneering studies which proved that vitamin D3 deficiency correlates directly with immune diseases such as multiple sclerosis (MS) (17), systemic lupus erythematosus (18), inflammatory bowel disease (IBD) (19), rheumatoid arthritis (20), and autoimmune thyroid disease (21). Vitamin D3 supplementation has been shown to reduce severity and incidence of such diseases not only in animal models but also in clinical studies (22). Based on studies which show that application of several vitamin D3 analogs has been effective in some solid organ transplantations (23–25), Pakkala and colleagues successfully achieved prevention of GvHD symptoms in a rat transplantation model by a 1,25(OH)2D3 analog (MC1288) (26). Further investigations proved that certain VDR polymorphisms are associated with higher risk of severe GvHD (27–29). Since patients receiving HSCT are malnourished, less exposed to sunlight and have an altered vitamin D3 metabolism due to medications and impaired organ function, they are predestined for vitamin D3 deficiency (30). In fact, Kreutz et al. demonstrated that conversion of 25(OH)D3 into 1,25(OH)2D3 is impaired in GvHD patients and that 25(OH)D3 serum levels were lower in grade III-IV than in grade I-II GvHD patients (31). The high prevalence of low vitamin D3 levels in patients undergoing HSCT is reported in other studies as well and might also be associated with a higher incidence of GvHD (32–34). These findings suggest a pivotal protective role of vitamin D3 in GvHD pathogenesis. Recently, Chen and Mayne reviewed the immunomodulatory effects of vitamin A and D in the context of GvHD (35). In the following, vitamin D3 will be analyzed briefly as an important modulator of both innate and adaptive immune system.
Molecular Actions of Vitamin D3 in the Innate Immune System of GvHD Patients
Antimicrobial Activities
Although the precise mechanisms of vitamin D3 remained unclear for a long time, patients infected with Mycobacterium tuberculosis (Mtb) have been treated with UVB irradation and cod liver oil in the pre-antibiotic era (36, 37). In 1980, Rook and colleagues could evidence that growth of Mtb was impeded in vitro by 1,25(OH)2D3 in human monocytes and macrophages (38, 39). Since then, it became increasingly clear that vitamin D3 exerts anti-microbial effects (40). Subsequent studies demonstrated that 1,25(OH)2D3 leads to release of anti-microbial peptides such as LL-37 and β-defensin (41–43). LL-37 is the cleavage product of human cathelicidin antimicrobial peptide (hCAP18, CAMP) and is known for its antibacterial function by inducing bacterial lysis and death (44). Upon infection, lung epithelial cells locally produce 1,25(OH)2D3 which in turn enhances LL-37 expression (45). Cathelicidin-deficient mice have been shown to be more susceptible to infections with Streptococcus, Pseudomonas, and E. coli (46). Cathelicidin does not only increase phagocytic activity of macrophages (47) but also promotes reactive oxygen species (ROS) production (48, 49), leading to direct antimicrobial effects. Moreover, cathelicidin triggers autophagy and reactivates phagolysosomal fusion in macrophages, which enhances degradation of intracellular pathogens such as Mtb, Salmonella, and Coxiella (14, 50). Even viral infections with influenza A (51) or fungal infections with Candida albicans (52) in mice are reduced by cathelicidin. Accumulating data have revealed that the intestinal barrier is supported by vitamin D3-dependent upregulation of tight junction proteins (53, 54), which is a fundamental requirement for efficient defense against pathogens. The loss of intestinal barrier function is also considered to be a driving factor for GvHD development (55). Thus, vitamin D3-dependent release of cathelicidin and the protection of epithelial barriers might improve graft- vs.-infection (GVI) effects in allo-HSCT patients.
Recent studies have now discovered a novel role of LL-37 in cancer (56) and inflammatory diseases (57). Strikingly, LL-37 does not only possess anti-microbial but also anti-inflammatory features, since it has been shown to inhibit the release of pro-inflammatory mediators such as TNF-α, IL-6, and IL-8 by neutrophils (48). Additionally, cathelicidin reduces mortality in mice infected with P. aeruginosa by neutralizing endotoxin-mediated inflammation (58).
Hence, vitamin D3-triggered activity of cathelicidin links anti-microbial and anti-inflammatory effects in the innate immune system. Since GvHD patients have an increased risk for severe infections due to immunosuppressive drugs (59), vitamin D3-mediated enhancement of antimicrobial defense mechanisms might reduce co-morbidity by infectious diseases. Therefore, it is conceivable that vitamin D3 might play an important and yet unrecognized role in GVI.
Anti-inflammatory Effects
As already mentioned, vitamin D3 elicits not only antimicrobial but also anti-inflammatory responses. Even though vitamin D3 enhances the maturation of human macrophages and their function as phagocytes (60), their capacity of antigen presentation and consequently also the priming of T cells is limited due to reduction of MHC-II expression (30, 61). Instead, 1,25(OH)2D3 polarizes macrophages toward an anti-inflammatory M2 subtype, which restrains colitis in mice (62). In humans and mice, vitamin D3 generates a tolerogenic phenotype and alters the cytokine and chemokine profile of mature DCs (mDCs) in vivo and in vitro, which are inhibited in differentiation, maturation and proliferation (63–65). In mixed lymphocyte reactions, proliferation of T cells, co-cultured with these 1,25(OH)2D3-induced tolerogenic DCs, was indirectly inhibited. Apart from preventing DCs to home into the lymph node by reducing CCR7-expression, vitamin D3 also decreases expression of the co-stimulatory molecules CD40, CD80 and CD86 and secretion of cytokines such as IL-6, IL-12, and TNF-α (66). Recently, Saul and colleagues revealed that CD31 is increasingly expressed on DCs, leading to impairment of cell-cell contact, which is essential for T cell priming (67). Moreover, secretion of immunosuppressive IL-10 is enhanced while IL-12 secretion by DCs is impaired, which leads to a weaker T helper Th1- and Th17- cell immune response (68). As a result, activation and differentiation of alloreactive CD4+ T cells is reduced in vitro (65). Furthermore, vitamin D3-treated DCs increase the frequency of suppressive CD4+CD25+ regulatory T cells (Treg) (69), which fosters peripheral tolerance to allografts (70). One study indicated that vitamin D3-mediated increase of CD4+Nrp-1+ cells ameliorates collagen-induced arthritis (71). Recently, Xu and colleagues established engineered DCs to de novo produce calcitriol in order to generate more gut-homing Tregs for efficient mitigation of intestinal inflammation (72). These results proved that 1,25(OH)2D3-induced tolerogenic DCs modulate T cells toward a regulatory and anti-inflammatory immune response in vivo and ameliorate acute GvHD (aGvHD) in mice (64, 73). Coussens and colleagues suggest that vitamin D3 supplementation in tuberculosis patients helps to restrict inflammatory responses by reducing circulating concentrations of chemokines such as CXCL9, CXCL10, and MMP-9 (74, 75). Additionally, upregulation of chemokine receptor CXCR3 fosters DC migration to inflammation spots (69). In vitro studies showed that Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling and inflammatory cytokines, such as IFN-γ, TNF-α, and Flt-3L, are significantly reduced in NK-cells upon vitamin D3-treatment or its analog seocalcitol (EB1089) (76). Interestingly, JAK1/2 have already been identified as potential therapeutic targets in GvHD since it was shown to reduce GvHD in mice while GvT could be preserved (77). Clinical trials verified that the JAK1/2 inhibitor Jakafi® (Ruxolitinib) reduces efficiently steroid-refractory GvHD (78, 79) and has recently been approved by the U.S. Food and Drug Administration (FDA).
Altogether, vitamin D3 modifies the innate immune system by exerting not only anti-microbial but also anti-inflammatory functions. Since GvHD patients often show co-morbidity of fungal, viral and bacterial infections (80, 81), increased infection rate as well as exaggerated inflammation are key issues needed to be combatted in this disease. Since persistence of APCs despite the conditioning regimen is the major cause of generation of alloreactive lymphocyte, manipulation of the innate immune system toward tolerogenic host-DCs by vitamin D3 in order to reduce their allo-stimulatory potential might help to prevent GvHD (82).
Effects of Vitamin D3 on the Adaptive Immune System
Apart from the above discussed indirect effects on T cells by vitamin D3-dependent modulation of innate immune cells, the hormone has also direct impacts on the adaptive immune system since T cells are known to express VDR, which enables them to respond to 1,25(OH)2D3 (83). Although the VDR appeared to be upregulated in activated alloreactive T cells indicating a role of vitamin D3 in T cell activation (30), studies proved that 1,25(OH)2D3 directly inhibits proliferation and IL-2 production of CD4+ T cells (84, 85). Similar to its effect on APCs, 1,25(OH)2D3 reduces expression of homing receptors such as CCR10 as well as secretion of IFN-γ and IL-10 by T cells (86). Especially Th1 cell proliferation is inhibited via the JAK/STAT signaling pathway (87), while Th2 cells are increased directly (88, 89). Therefore, vitamin D3 alters the T cell immunity by transforming Th1- and Th17-responses toward an anti-inflammatory Th2-activity. This mechanism is even amplified since expression of CYP27B1 is also enhanced in activated lymphocytes (30). CD8+ T cells are inhibited in proliferation in vitro and in vivo by vitamin D3 (90). It is documented that vitamin D3 inhibits pro-inflammatory T cells in IBD patients (91). Since IBD pathogenesis is driven by loss of intestinal barrier function, clinical manifestations of IBD resemble GvHD symptoms in the gastrointestinal tract (55). Such parallels suggest that vitamin D3 might achieve similar effects in allo-HSCT. However, 1,25(OH)2D3 does not only affect T cells but also modulates differentiation and antibody-production of B cells (92). In addition, it induces apoptosis and cell cycle arrest of proliferating B cells resulting in impaired plasma cell differentiation and less autoantibody expression (93).
Altogether, vitamin D3 has an overall anti-microbial and anti-inflammatory effect on both innate and adaptive immune system. Therefore, vitamin D3 could be a potent supplementary agent in GvHD patients which might improve the patient's life quality by decreasing infectious- and inflammation-mediated co-morbidity.
Potential GvL-effects Mediated by Vitamin D3
As mentioned earlier, it is pivotal to preserve the immune balance by avoiding alloreactivity of donor T cells against healthy tissue while still maintaining their anti-tumorigenic effect. Interestingly, vitamin D3 does not only reduce harmful GvHD effects but also exerts anti-tumor activity. So far, scientific literature supporting this assumption in the transplantation setting remains sparse. However, a few studies provide indications for its hypothetical anti-cancer effects. In their retrospective study, Radujkovic et al. could demonstrate that pre-transplant vitamin D3 deficiency in patients diagnosed with myeloid malignancies correlates with a higher risk of relapse mortality (94). To our knowledge, only three other studies have also investigated this association (90, 95, 96). So far, only one study included few patients which underwent autologous transplantation (97). However, they only figured out that sufficient vitamin D3 levels are hard to achieve. In summary, these data suggest that prospective randomized trials have to prove whether vitamin D3 supplementation during stem cell transplantation could enhance GvL effects.
Vitamin D3 and Cancer
The first correlation of solar radiation and cancer was initially suggested by Apperly in 1941, who attributed sunlight radiation a protective role against many types of cancer except skin cancer (98). Decades later, Colston et al. were the first ones to show a dose-dependent inhibitory effect of 1,25(OH)2D3 on melanoma cells (99, 100). Epidemiological studies provide evidence that poor sunlight exposure and vitamin D3 deficiency correlate directly with incidence as well as mortality rate of several cancer types. These findings suggest a protective role of vitamin D3 in carcinogenesis (101). Accumulating studies have revealed that 1,25(OH)2D3 suppresses tumor growth (102–104) and exhibits anti-proliferative activities in squamous cell carcinoma (105), prostate (106), breast (107, 108), lung (109), head and neck cancer (110) and hematologic malignancies such as Hodgkin's lymphoma (111) or chronic lymphocytic leukemia (CLL) (112). In colorectal cancer, a clinical trial provides evidence that 1,25(OH)2D3 supplementation can efficiently reduce the risk of tumor development (113). However, other epidemiological studies report contradictory results (114–116), which might be a result of using supra-physiological concentrations of calcitriol (117), VDR gene polymorphisms (118), lack of control groups or inappropriate dosage and administration of vitamin D3.
Apart from the discovery that sufficient vitamin D3 supplementation could help to prevent cancer pathogenesis, numerous in vitro and in vivo studies provide evidence that 1,25(OH)2D3 and its analogs could reduce tumor growth and might be used as potential anticancer agent (15, 119–121). Supporting this, animal studies report that VDR-deletion in mice makes them more susceptible to chemical induced carcinogenesis in epidermis, lymphoid and mammary tissue (122). Interestingly, life expectancy of leukemic mice could be prolonged by treatment with a 1,25(OH)2D3 analog (123). A chemoprevention study revealed that 1,25(OH)2D3-treated Nkx3.1;Pten mutant mice show retarded development of neoplasias when it was administered during early-stage carcinogenesis (124).
There is clear evidence that cancer cells exploit and dysregulate the vitamin D3 metabolism enabling them to escape its cancer protective role (15). CYP24A1 has been shown to be overexpressed in cancer cells while activity of CYP27B1 is reduced in human prostate cancer cells (125, 126). Furthermore, CYP24A1 was identified as potential oncogene in breast cancer and elevated expression of VDR in tissues of breast and prostate cancer correlates with better prognosis of survival (127).
Mechanisms of Anti-tumorigenic Actions
Although the precise mechanisms of vitamin D3-mediated anti-tumorigenic action are not yet fully understood, it has been postulated that vitamin D3 modulates gene expression involved in apoptosis, cell cycle and autophagy in tumor cells (128). Apoptosis is initiated due to downregulation of anti-apoptotic protein Bcl2 while expression of pro-apoptotic proteins increases (129). Jiang et al. suggest that 1,25(OH)2D3 induces cell death by degrading telomerase reverse transcriptase (TERT) mRNA and thus reduces telomerase activity (130). 1,25(OH)2D3-induced upregulation of p21 and p27, which are cyclin-dependent kinase (CDK) inhibitors, induces cell cycle arrest (121, 129, 131). Furthermore, vitamin D3 mediates anti-proliferative activity by enhancing expression of Dickkopf-1 (DKK-1), which is an antagonist in the Wnt/β-catenin signaling pathway (132). In vitro as well as in vivo studies report inhibition of proliferation and angiogenesis by vitamin D3. It suppresses hypoxia-inducible factor 1-alpha (HIF1A) leading to reduced expression of vascular endothelial growth factor (VEGF) and thereby inhibition of angiogenesis (133, 134). Autophagy is not only triggered in infected macrophages, but also in tumor cells such as breast cancer. Since autophagy appears to protect healthy tissue from cancer initiation, vitamin D3-treatment might contribute to suppression of carcinogenesis (135). It also increases activity of antioxidant enzymes such as superoxide dismutase 1/2 (SOD1/2) and therefore protects DNA from ROS-induced damage (129). Upon vitamin D3 administration, DNA damage repair proteins, such as p53, are upregulated in vitro (15).
Strikingly, anti-tumor activity of tumor-associated macrophages (TAMs) against lymphomas has been shown to be enhanced by vitamin D3-triggered increase of antibody-dependent cellular toxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) (136). Current observations of Busch et al. reveal that combination of vitamin D3 with immunomodulatory drugs (IMiDs), such as lenalidomide, helps to restore the defective vitamin D3 metabolism in myeloma-associated macrophages and improves cytotoxicity against multiple myeloma cells mediated by specific anti-CD38 antibodies such as MOR202 (137, 138). Furthermore, exosomal transfer of microRNAs, which induce tumor-promoting myeloid-derived suppressor cells, was impeded by vitamin D3 (139). The previously mentioned cathelicidin, which is secreted by human macrophages, has also been shown to mediate direct anti-tumor efficacy against high-grade B cell lymphoma by increasing ADCC (136). In summary, there is strong evidence that vitamin D3 exerts direct anti-tumorigenic functions which might be applicable in allo-HSCT patients in order to boost GvL effects.
Mediation of Anti-inflammation to Antagonize Carcinogenesis
In 1863, Virchow postulated for the first time that tissue proliferation and hence tumor progression might be provoked by an inflammatory microenvironment connecting cancer with inflammation (140, 141). Since inflammatory tissue provides ideal conditions for genetic mutations, it seems obvious that tumor progression occurs more frequently in inflammatory environment than in healthy tissue. Clinical studies proved that localized persistent inflammation is a risk factor for the development of cancer in adjacent organs, e.g., patients with ulcerative colitis have a higher incidence of colorectal cancer (142). Given that inflammation promotes carcinogenesis, vitamin D3-dependent anti-inflammatory activity could reduce tumor progression. In esophageal squamous cell carcinoma, 1,25(OH)2D3 impedes tumor growth by inhibition of IL-6 signaling (117). Accumulating data report that 1,25(OH)2D3 inhibits prostaglandin (PG) (143), p38 MAPK (144) and nuclear factor kappa B (NFκB) signaling pathways (15). Although there is increasing evidence for inflammation-driven carcinogenesis, not every type of chronic inflammation evokes tumor development, which appears to be contradictory.
Despite the well-founded evidence of anti-tumorigenic effects of vitamin D3 in solid tumors, studies on hematological malignancies remain elusive. Given that vitamin D3 deficiency correlates with worse relapse-free survival (94–96) and the known anti-tumorigenic effects of vitamin D3, one might think that it could also enhance GvL. By using mice fed with low and high vitamin D3 doses or by performing clinical trials with vitamin D3 supplementation, the actual effect on GvL could be investigated.
Conclusion/Perspectives
In summary, we assume that vitamin D3 could be a potential immune modulating agent for supplementation before and during allo-HSCT. It is conceivable that vitamin D3 might be able to maintain and improve the patient's immune balance and epithelial barrier function. Mounting evidence indicates that vitamin D3 could alleviate GvHD by enhancing anti-inflammatory responses while it might coincidently ameliorate GvI effects due to its anti-microbial activities. Moreover, GvL might be boosted because vitamin D3 could at least reinforce anti-tumorigenic responses of myeloid cells (Figure 2).
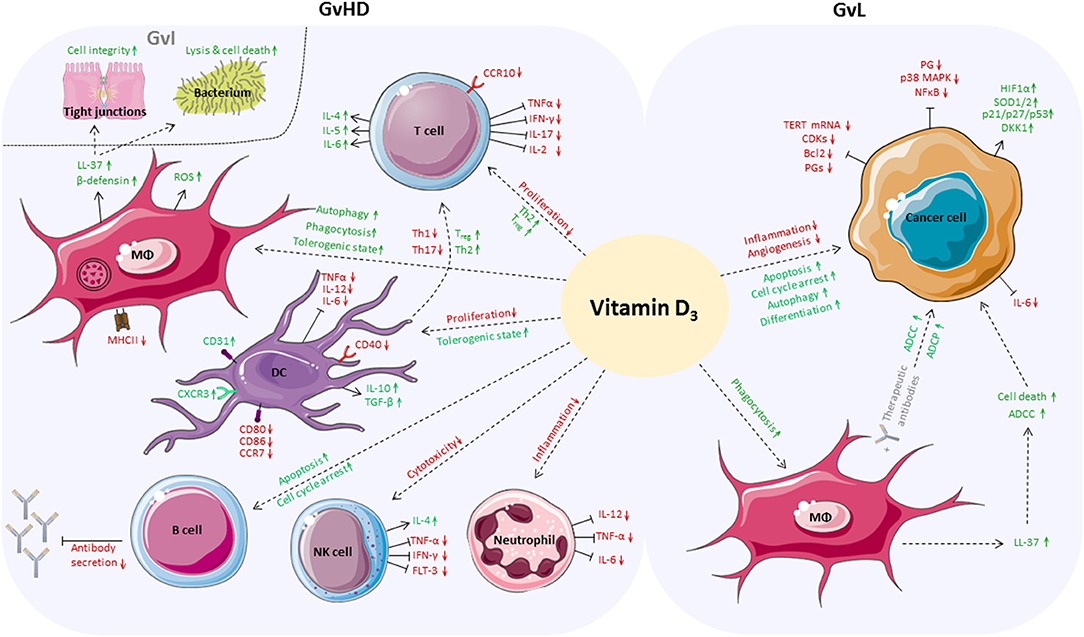
Figure 2. Positive effects of vitamin D3 in the allo-HSCT setting. Vitamin D3 affects a broad variety of cells of the adaptive and innate immune system which play an important role in boosting GvL responses while alleviating GvHD. On side of GvHD, proliferation of T cells is inhibited and anti-inflammatory differentiation is favored. Vitamin D3 enhances autophagy, phagocytic activity and tolerogenic capacity of macrophages. Vitamin D3-dependent release of antimicrobial peptides by macrophages fortifies GvI effects, increases cell integrity, and induces cell death of bacteria. DCs are inhibited in proliferation and reduce Th1 and Th17 responses while the Treg population increases. Vitamin D3 triggers apoptosis and cell cycle arrest of B cells and inhibits antibody secretion. Vitamin D3 reduces cytotoxic activity of NK cells and impedes neutrophils to secrete pro-inflammatory cytokines. In case of GvL, apoptosis, autophagy and differentiation of cancer cells are directly enhanced by vitamin D3, whereas inflammation and angiogenesis are reduced. Vitamin D3 enhances ADCC and ADCP of cancer cells by macrophages directly or by release of LL-37.
Besides its easy availability, economy and role in preserving the intestinal barrier integrity (53), vitamin D3 helps to maintain calcium and bone homeostasis and hence prevents osteoporosis. Given that allo-HSCT patients often suffer from bone loss upon conditioning regimens, immunosuppressive treatment and immobilization, it might also improve GvHD by preventing osteoporosis (34). Cholecalciferol can usually be administered safely in high doses without occurrence of abnormal calcium metabolism (145). However, sufficient vitamin D3 levels cannot be achieved in all patients despite high-dose supplementation (97). Therefore, treatment with 1,25(OH)2D3 might be the more efficient version. However, the probably greatest restraining factor of 1,25(OH)2D3 is its dose-limiting toxicity causing hypercalcemia and hypercalciuria. One possible solution might be the administration of 1,25(OH)2D3 analogs, some of which have already been shown to be less calcemic (146, 147).
Until now, only few clinical trials with vitamin D3 in the allo-HSCT setting have been conducted and have shown effective outcomes (90). The most recent study of Carillo-Cruz et al. suggests that universal vitamin D3 medication remains challenging due to VDR polymorphisms (29). Our hypothesis that vitamin D3 could improve GvL might seem controversial due to its known anti-inflammatory activities on T cells. However, it was shown by Essen et al. that TCR signaling in naïve human T cells induces VDR expression (148). This in turn results in upregulated PLC-γ1 expression and thus higher activation and priming of naïve T cells. Although there is evidence that vitamin D3 attenuates IL-6 signaling in human esophageal squamous cell carcinoma (SCC) cell lines (117), the in vivo study of Bendix-Struve and colleagues demonstrated that T cells of vitamin D3-supplemented Crohn's disease (CD) patients produced more IL-6 (149). Proliferation of CD4+ T cells was higher in vitamin D3-treated patients compared to the placebo group. Additionally, VDR was shown to be important for the development of CD8αα+ TCRαβ+ cells, which help to maintain tolerance in the gut and suppress intestinal inflammation (150). Expression of the gut-homing receptor CCR9 is suppressed in T cells upon vitamin D3 stimulation, which might prevent homing of potential alloreactive T cells into the gut. However, these data provide only indications that vitamin D3 might promote GvL despite its anti-inflammatory properties.
In conclusion, prospective in vivo studies in humans are inevitable to investigate the efficacy of vitamin D3 supplementation and to achieve approved clinical application.
Author Contributions
CF wrote the manuscript and created the figures. HB took the lead in writing the manuscript. MK and KP provided critical feedback, contributed to the final version of the manuscript, and approved it for publication.
Funding
HB was supported by Wilhelm-Sander Foundation. HB, CF, KP, and MK were funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – B12- TRR 221.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Illustrations for figures were obtained from https://smart.servier.com.
References
1. Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. (2015) 21:1863–9. doi: 10.1016/j.bbmt.2015.07.032
2. Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. (2008) 112:4371–83. doi: 10.1182/blood-2008-03-077974
3. Ogonek J, Kralj Juric M, Ghimire S, Varanasi PR, Holler E, Greinix H, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol. (2016) 7:507. doi: 10.3389/fimmu.2016.00507
4. Li JM, Giver CR, Lu Y, Hossain MS, Akhtari M, Waller EK. Separating graft-versus-leukemia from graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Immunotherapy. (2009) 1:599–621.
5. Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. (2012) 18:1150–63. doi: 10.1016/j.bbmt.2012.04.005
6. Datta A, David R, Glennie S, Scott D, Cernuda-Morollon E, Lechler RI, et al. Differential effects of immunosuppressive drugs on T-cell motility. Am J Transplant. (2006) 6:2871–83. doi: 10.1111/j.1600-6143.2006.01553.x
7. Adorini L, Giarratana N, Penna G. Pharmacological induction of tolerogenic dendritic cells and regulatory T cells. Semin Immunol. (2004) 16:127–34. doi: 10.1016/j.smim.2003.12.008
8. Chang YJ, Zhao XY, Huang XJ. Strategies for enhancing and preserving anti-leukemia effects without aggravating graft-versus-host disease. Front Immunol. (2018) 9:3041. doi: 10.3389/fimmu.2018.03041
9. Deluca HF. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. (2014) 3:479. doi: 10.1038/bonekey.2013.213
10. Kreutz M, Andreesen R, Krause SW, Szabo A, Ritz E, Reichel H. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood. (1993) 82:1300–7. doi: 10.1182/blood.V82.4.1300.1300
11. Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. (2003) 102:3314–6. doi: 10.1182/blood-2002-11-3521
12. Gottfried E, Rehli M, Hahn J, Holler E, Andreesen R, Kreutz M. Monocyte-derived cells express CYP27A1 and convert vitamin D3 into its active metabolite. Biochem Biophys Res Commun. (2006) 349:209–13. doi: 10.1016/j.bbrc.2006.08.034
13. Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. (2013) 5:2502–21. doi: 10.3390/nu5072502
14. Bruns H, Stenger S. New insights into the interaction of Mycobacterium tuberculosis and human macrophages. Future Microbiol. (2014) 9:327–41. doi: 10.2217/fmb.13.164
15. Jeon SM, Shin EA. Exploring vitamin D metabolism and function in cancer. Exp Mol Med. (2018) 50:20. doi: 10.1038/s12276-018-0038-9
16. Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med. (2010) 88:441–50. doi: 10.1007/s00109-010-0590-9
17. Munger KL, Ascherio A. Prevention and treatment of MS: studying the effects of vitamin Mult Scler D. (2011) 17:1405–11. doi: 10.1177/1352458511425366
18. Hassanalilou T, Khalili L, Ghavamzadeh S, Shokri A, Payahoo L, Bishak YK. Role of vitamin D deficiency in systemic lupus erythematosus incidence and aggravation. Auto Immun Highlights. (2017) 9:1. doi: 10.1007/s13317-017-0101-x
19. Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med. (2004) 229:1136–42. doi: 10.1177/153537020422901108
20. Yang J, Liu L, Zhang Q, Li M, Wang J. Effect of vitamin D on the recurrence rate of rheumatoid arthritis. Exp Ther Med. (2015) 10:1812–6. doi: 10.3892/etm.2015.2747
21. Kim D. The role of vitamin D in thyroid diseases. Int J Mol Sci. (2017) 18:1949. doi: 10.3390/ijms18091949
22. Czaja AJ, Montano-Loza AJ. Evolving role of vitamin D in immune-mediated disease and its implications in autoimmune hepatitis. Dig Dis Sci. (2019) 64:324–44. doi: 10.1007/s10620-018-5351-6
23. Johnsson C, Tufveson G. MC 1288–a vitamin D analogue with immunosuppressive effects on heart and small bowel grafts. Transpl Int. (1994) 7:392–7. doi: 10.1007/BF00346032
24. Lewin E, Olgaard K. The in vivo effect of a new, in vitro, extremely potent vitamin D3 analog KH1060 on the suppression of renal allograft rejection in the rat. Calcif Tissue Int. (1994) 54:150–4. doi: 10.1007/BF00296066
25. Hullett DA, Cantorna MT, Redaelli C, Humpal-Winter J, Hayes CE, Sollinger HW, et al. Prolongation of allograft survival by 1,25-dihydroxyvitamin D3. Transplantation. (1998) 66:824–8. doi: 10.1097/00007890-199810150-00002
26. Pakkala I, Taskinen E, Pakkala S, Raisanen-Sokolowski A. MC1288, a vitamin D analog, prevents acute graft-versus-host disease in rat bone marrow transplantation. Bone Marrow Transplant. (2001) 27:863–7. doi: 10.1038/sj.bmt.1702873
27. Middleton PG, Cullup H, Dickinson AM, Norden J, Jackson GH, Taylor PR, et al. Vitamin D receptor gene polymorphism associates with graft-versus-host disease and survival in HLA-matched sibling allogeneic bone marrow transplantation. Bone Marrow Transplant. (2002) 30:223–8. doi: 10.1038/sj.bmt.1703629
28. Rocha V, Porcher R, Fernandes JF, Filion A, Bittencourt H, Silva W Jr, et al. Association of drug metabolism gene polymorphisms with toxicities, graft-versus-host disease and survival after HLA-identical sibling hematopoietic stem cell transplantation for patients with leukemia. Leukemia. (2009) 23:545–56. doi: 10.1038/leu.2008.323
29. Carrillo-Cruz E, Garcia-Lozano JR, Marquez-Malaver FJ, Sanchez-Guijo FM, Montero Cuadrado I, Ferra ICC, et al. Vitamin D modifies the incidence of graft-versus-host disease after allogeneic stem cell transplantation depending on the vitamin D receptor (VDR) polymorphisms. Clin Cancer Res. (2019) 25:4616–23. doi: 10.1158/1078-0432.CCR-18-3875
30. Ros-Soto J, Anthias C, Madrigal A, Snowden JA. Vitamin D: is it important in haematopoietic stem cell transplantation? A review. Bone Marrow Transplant. (2018) 54:810–20. doi: 10.1038/s41409-018-0377-0
31. Kreutz M, Eissner G, Hahn J, Andreesen R, Drobnik W, Holler E. Variations in 1 alpha,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 serum levels during allogeneic bone marrow transplantation. Bone Marrow Transplant. (2004) 33:871–3. doi: 10.1038/sj.bmt.1704448
32. Beebe K, Magee K, McNulty A, Stahlecker J, Salzberg D, Miller H, et al. Vitamin D deficiency and outcomes in pediatric hematopoietic stem cell transplantation. Pediatr Blood Cancer. (2018) 65:e26817. doi: 10.1002/pbc.26817
33. Glotzbecker B, Ho VT, Aldridge J, Kim HT, Horowitz G, Ritz J, et al. Low levels of 25-hydroxyvitamin D before allogeneic hematopoietic SCT correlate with the development of chronic GVHD. Bone Marrow Transplant. (2013) 48:593–7. doi: 10.1038/bmt.2012.177
34. Sproat L, Bolwell B, Rybicki L, Dean R, Sobecks R, Pohlman B, et al. Vitamin D level after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. (2011) 17:1079–83. doi: 10.1016/j.bbmt.2010.12.704
35. Chen X, Mayne CG. The role of micronutrients in graft-vs.-host disease: immunomodulatory effects of vitamins A and D. Front Immunol. (2018) 9:2853. doi: 10.3389/fimmu.2018.02853
36. Brighenti S, Bergman P, Martineau AR. Vitamin D and tuberculosis: where next? J Intern Med. (2018) 284:145–62. doi: 10.1111/joim.12777
38. Rook GA, Steele J, Fraher L, Barker S, Karmali R, O'Riordan J, et al. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. (1986) 57:159–63.
39. Crowle AJ, Ross EJ, May MH. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. (1987) 55:2945–50. doi: 10.1016/0041-3879(88)90036-0
40. Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. (2011) 3:104ra102. doi: 10.1126/scitranslmed.3003045
41. Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. (2004) 173:2909–12. doi: 10.4049/jimmunol.173.5.2909
42. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. (2005) 19:1067–77. doi: 10.1096/fj.04-3284com
43. Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. (2007) 178:7190–8. doi: 10.4049/jimmunol.178.11.7190
44. Leszczynska K, Namiot D, Byfield FJ, Cruz K, Zendzian-Piotrowska M, Fein DE, et al. Antibacterial activity of the human host defence peptide LL-37 and selected synthetic cationic lipids against bacteria associated with oral and upper respiratory tract infections. J Antimicrob Chemother. (2013) 68:610–8. doi: 10.1093/jac/dks434
45. Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. (2008) 181:7090–9. doi: 10.4049/jimmunol.181.10.7090
46. van der Does AM, Bergman P, Agerberth B, Lindbom L. Induction of the human cathelicidin LL-37 as a novel treatment against bacterial infections. J Leukoc Biol. (2012) 92:735–42. doi: 10.1189/jlb.0412178
47. Wan M, van der Does AM, Tang X, Lindbom L, Agerberth B, Haeggstrom JZ. Antimicrobial peptide LL-37 promotes bacterial phagocytosis by human macrophages. J Leukoc Biol. (2014) 95:971–81. doi: 10.1189/jlb.0513304
48. Alalwani SM, Sierigk J, Herr C, Pinkenburg O, Gallo R, Vogelmeier C, et al. The antimicrobial peptide LL-37 modulates the inflammatory and host defense response of human neutrophils. Eur J Immunol. (2010) 40:1118–26. doi: 10.1002/eji.200939275
49. Choi H, Yang Z, Weisshaar JC. Oxidative stress induced in E. coli by the human antimicrobial peptide LL-37. PLoS Pathog. (2017) 13:e1006481. doi: 10.1371/journal.ppat.1006481
50. Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. (2009) 6:231–43. doi: 10.1016/j.chom.2009.08.004
51. Barlow PG, Svoboda P, Mackellar A, Nash AA, York IA, Pohl J, et al. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE. (2011) 6:e25333. doi: 10.1371/journal.pone.0025333
52. Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. (2005) 125:108–15. doi: 10.1111/j.0022-202X.2005.23713.x
53. Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. (2008) 294:G208–16. doi: 10.1152/ajpgi.00398.2007
54. Zhang YG, Wu S, Lu R, Zhou D, Zhou J, Carmeliet G, et al. Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci Rep. (2015) 5:10642. doi: 10.1038/srep10642
55. Nalle SC, Turner JR. Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol. (2015) 8:720–30. doi: 10.1038/mi.2015.40
56. Chen X, Zou X, Qi G, Tang Y, Guo Y, Si J, et al. Roles and mechanisms of human cathelicidin LL-37 in cancer. Cell Physiol Biochem. (2018) 47:1060–73. doi: 10.1159/000490183
57. Sun L, Wang W, Xiao W, Yang H. The roles of cathelicidin LL-37 in inflammatory bowel disease. Inflamm Bowel Dis. (2016) 22:1986–91. doi: 10.1097/MIB.0000000000000804
58. Kirikae T, Hirata M, Yamasu H, Kirikae F, Tamura H, Kayama F, et al. Protective effects of a human 18-kilodalton cationic antimicrobial protein (CAP18)-derived peptide against murine endotoxemia. Infect Immun. (1998) 66:1861–8.
59. Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. (2009) 373:1550–61. doi: 10.1016/S0140-6736(09)60237-3
60. Kreutz M, Andreesen R. Induction of human monocyte to macrophage maturation in vitro by 1,25-dihydroxyvitamin D3. Blood. (1990) 76:2457–61. doi: 10.1182/blood.V76.12.2457.bloodjournal76122457
61. Rigby WF, Waugh M, Graziano RF. Regulation of human monocyte HLA-DR and CD4 antigen expression, and antigen presentation by 1,25-dihydroxyvitamin D3. Blood. (1990) 76:189–97. doi: 10.1182/blood.V76.1.189.bloodjournal761189
62. Zhu X, Zhu Y, Li C, Yu J, Ren D, Qiu S, et al. 1,25Dihydroxyvitamin D regulates macrophage polarization and ameliorates experimental inflammatory bowel disease by suppressing miR-125b. Int Immunopharmacol. (2019) 67:106–18. doi: 10.1016/j.intimp.2018.12.015
63. Berer A, Stockl J, Majdic O, Wagner T, Kollars M, Lechner K, et al. 1,25-Dihydroxyvitamin D(3) inhibits dendritic cell differentiation and maturation in vitro. Exp Hematol. (2000) 28:575–83. doi: 10.1016/S0301-472X(00)00143-0
64. Ferreira GB, Gysemans CA, Demengeot J, da Cunha JP, Vanherwegen AS, Overbergh L, et al. 1,25-Dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J Immunol. (2014) 192:4210–20. doi: 10.4049/jimmunol.1302350
65. Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. (2000) 164:2405–11. doi: 10.4049/jimmunol.164.5.2405
66. Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. (2009) 45:190–7. doi: 10.1016/j.cyto.2008.12.009
67. Saul L, Mair I, Ivens A, Brown P, Samuel K, Campbell JDM, et al. 1,25-Dihydroxyvitamin D3 Restrains CD4(+) T Cell Priming Ability of CD11c(+) Dendritic Cells by Upregulating Expression of CD31. Front Immunol. (2019) 10:600. doi: 10.3389/fimmu.2019.00600
68. D'Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. (1998) 101:252–62. doi: 10.1172/JCI1050
69. Vanherwegen AS, Cook DP, Ferreira GB, Gysemans C, Mathieu C. Vitamin D-modulated dendritic cells delay lethal graft-versus-host disease through induction of regulatory T cells. J Steroid Biochem Mol Biol. (2019) 188:03–10. doi: 10.1016/j.jsbmb.2018.12.013
70. Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. (2001) 167:1945–53. doi: 10.4049/jimmunol.167.4.1945
71. Zhou L, Wang J, Li J, Li T, Chen Y, June RR, et al. 1,25-Dihydroxyvitamin D3 ameliorates collagen-induced arthritis via suppression of Th17 cells through miR-124 mediated inhibition of IL-6 signaling. Front Immunol. (2019) 10:178. doi: 10.3389/fimmu.2019.00178
72. Xu Y, Cheng Y, Baylink DJ, Wasnik S, Goel G, Huang M, et al. in vivo generation of gut-homing regulatory T cells for the suppression of colitis. J Immunol. (2019) 202:3447–57. doi: 10.4049/jimmunol.1800018
73. Vanherwegen AS, Eelen G, Ferreira GB, Ghesquiere B, Cook DP, Nikolic T, et al. Vitamin D controls the capacity of human dendritic cells to induce functional regulatory T cells by regulation of glucose metabolism. J Steroid Biochem Mol Biol. (2019) 187:134–45. doi: 10.1016/j.jsbmb.2018.11.011
74. Coussens A, Timms PM, Boucher BJ, Venton TR, Ashcroft AT, Skolimowska KH, et al. 1alpha,25-dihydroxyvitamin D3 inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology. (2009) 127:539–48. doi: 10.1111/j.1365-2567.2008.03024.x
75. Coussens AK, Wilkinson RJ, Hanifa Y, Nikolayevskyy V, Elkington PT, Islam K, et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci USA. (2012) 109:15449–54. doi: 10.1073/pnas.1200072109
76. Olson KC, Kulling Larkin PM, Signorelli R, Hamele CE, Olson TL, Conaway MR, et al. Vitamin D pathway activation selectively deactivates signal transducer and activator of transcription (STAT) proteins and inflammatory cytokine production in natural killer leukemic large granular lymphocytes. Cytokine. (2018) 111:551–62. doi: 10.1016/j.cyto.2018.09.016
77. Carniti C, Gimondi S, Vendramin A, Recordati C, Confalonieri D, Bermema A, et al. Pharmacologic inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin Cancer Res. (2015) 21:3740–9. doi: 10.1158/1078-0432.CCR-14-2758
78. Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. (2015) 29:2062–8. doi: 10.1038/leu.2015.212
79. Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. (2014) 123:3832–42. doi: 10.1182/blood-2013-12-543736
80. Young JA. Infectious complications of acute and chronic GVHD. Best Pract Res Clin Haematol. (2008) 21:343–56. doi: 10.1016/j.beha.2008.02.017
81. Fuji S, Kapp M, Einsele H. Possible implication of bacterial infection in acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Front Oncol. (2014) 4:89. doi: 10.3389/fonc.2014.00089
82. Rosenblatt J, Bissonnette A, Ahmad R, Wu Z, Vasir B, Stevenson K, et al. Immunomodulatory effects of vitamin D: implications for GVHD. Bone Marrow Transplant. (2010) 45:1463–8. doi: 10.1038/bmt.2009.366
83. Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. (1983) 221:1181–3. doi: 10.1126/science.6310748
84. Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. (2009) 183:5458–67. doi: 10.4049/jimmunol.0803217
85. Vanham G, Ceuppens JL, Bouillon R. T lymphocytes and their CD4 subset are direct targets for the inhibitory effect of calcitriol. Cell Immunol. (1989) 124:320–33. doi: 10.1016/0008-8749(89)90134-2
86. Baeke F, Korf H, Overbergh L, van Etten E, Verstuyf A, Gysemans C, et al. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol. (2010) 121:221–7. doi: 10.1016/j.jsbmb.2010.03.037
87. Zhang Z, Chen F, Li J, Luo F, Hou T, Xu J, et al. 1,25(OH)2D3 suppresses proinflammatory responses by inhibiting Th1 cell differentiation and cytokine production through the JAK/STAT pathway. Am J Transl Res. (2018) 10:2737–46.
88. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. (2001) 167:4974–80. doi: 10.4049/jimmunol.167.9.4974
89. Hayes CE, Nashold FE, Spach KM, Pedersen LB. The immunological functions of the vitamin D endocrine system. Cell Mol Biol. (2003) 49:277–300.
90. Caballero-Velazquez T, Montero I, Sanchez-Guijo F, Parody R, Saldana R, Valcarcel D, et al. Immunomodulatory effect of vitamin D after allogeneic stem cell transplantation: results of a prospective multicenter clinical trial. Clin Cancer Res. (2016) 22:5673–81. doi: 10.1158/1078-0432.CCR-16-0238
91. Schardey J, Globig AM, Janssen C, Hofmann M, Manegold P, Thimme R, et al. Vitamin D inhibits pro-inflammatory T cell function in patients with inflammatory bowel disease. J Crohns Colitis. (2019). doi: 10.1093/ecco-jcc/jjz090. [Epub ahead of print].
92. Iho S, Takahashi T, Kura F, Sugiyama H, Hoshino T. The effect of 1,25-dihydroxyvitamin D3 on in vitro immunoglobulin production in human B cells. J Immunol. (1986) 136:4427–31.
93. Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. (2007) 179:1634–47. doi: 10.4049/jimmunol.179.3.1634
94. Radujkovic A, Kordelas L, Krzykalla J, Beelen DW, Benner A, Lehners N, et al. Pretransplant vitamin D deficiency is associated with higher relapse rates in patients allografted for myeloid malignancies. J Clin Oncol. (2017) 35:3143–52. doi: 10.1200/JCO.2017.73.0085
95. Hansson ME, Norlin AC, Omazic B, Wikstrom AC, Bergman P, Winiarski J, et al. Vitamin d levels affect outcome in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2014) 20:1537–43. doi: 10.1016/j.bbmt.2014.05.030
96. Lee HJ, Muindi JR, Tan W, Hu Q, Wang D, Liu S, et al. Low 25(OH) vitamin D3 levels are associated with adverse outcome in newly diagnosed, intensively treated adult acute myeloid leukemia. Cancer. (2014) 120:521–9. doi: 10.1002/cncr.28368
97. Wallace G, Jodele S, Myers KC, Dandoy CE, El-Bietar J, Nelson A, et al. Vitamin D deficiency in pediatric hematopoietic stem cell transplantation patients despite both standard and aggressive supplementation. Biol Blood Marrow Transplant. (2016) 22:1271–4. doi: 10.1016/j.bbmt.2016.03.026
98. Bertino JR. Landmark study: the relation of solar radiation to cancer mortality in North America. Cancer Res. (2016) 76:185. doi: 10.1158/0008-5472.CAN-15-3169
99. Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. (1981) 108:1083–6. doi: 10.1210/endo-108-3-1083
100. Giammanco M, Di Majo D, La Guardia M, Aiello S, Crescimannno M, Flandina C, et al. Vitamin D in cancer chemoprevention. Pharm Biol. (2015) 53:1399–434. doi: 10.3109/13880209.2014.988274
101. Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. (2009) 19:468–83. doi: 10.1016/j.annepidem.2009.03.021
102. Kasiappan R, Shen Z, Tse AK, Jinwal U, Tang J, Lungchukiet P, et al. 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and human cancer growth through microRNA-498. J Biol Chem. (2012) 287:41297–309. doi: 10.1074/jbc.M112.407189
103. Frampton RJ, Omond SA, Eisman JA. Inhibition of human cancer cell growth by 1,25-dihydroxyvitamin D3 metabolites. Cancer Res. (1983) 43:4443–7.
104. Honma Y, Hozumi M, Abe E, Konno K, Fukushima M, Hata S, et al. 1 alpha,25-Dihydroxyvitamin D3 and 1 alpha-hydroxyvitamin D3 prolong survival time of mice inoculated with myeloid leukemia cells. Proc Natl Acad Sci USA. (1983) 80:201–4. doi: 10.1073/pnas.80.1.201
105. Bernardi RJ, Johnson CS, Modzelewski RA, Trump DL. Antiproliferative effects of 1alpha,25-dihydroxyvitamin D(3) and vitamin D analogs on tumor-derived endothelial cells. Endocrinology. (2002) 143:2508–14. doi: 10.1210/endo.143.7.8887
106. Getzenberg RH, Light BW, Lapco PE, Konety BR, Nangia AK, Acierno JS, et al. Vitamin D inhibition of prostate adenocarcinoma growth and metastasis in the Dunning rat prostate model system. Urology. (1997) 50:999–1006. doi: 10.1016/S0090-4295(97)00408-1
107. Colston KW, Chander SK, Mackay AG, Coombes RC. Effects of synthetic vitamin D analogues on breast cancer cell proliferation in vivo and in vitro. Biochem Pharmacol. (1992) 44:693–702. doi: 10.1016/0006-2952(92)90405-8
108. Ooi LL, Zhou H, Kalak R, Zheng Y, Conigrave AD, Seibel MJ, et al. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Res. (2010) 70:1835–44. doi: 10.1158/0008-5472.CAN-09-3194
109. Higashimoto Y, Ohata M, Nishio K, Iwamoto Y, Fujimoto H, Uetani K, et al. 1 alpha, 25-dihydroxyvitamin D3 and all-trans-retinoic acid inhibit the growth of a lung cancer cell line. Anticancer Res. (1996) 16:2653–9.
110. Kornfehl J, Formanek M, Temmel A, Knerer B, Willheim M. Antiproliferative effects of the biologically active metabolite of vitamin D3 (1,25 [OH]2 D3) on head and neck squamous cell carcinoma cell lines. Eur Arch Otorhinolaryngol. (1996) 253:341–4. doi: 10.1007/BF00178289
111. Gharbaran R, Zhang B, Valerio L, Onwumere O, Wong M, Mighty J, et al. Effects of vitamin D3 and its chemical analogs on the growth of Hodgkin's lymphoma, in vitro. BMC Res Notes. (2019) 12:216. doi: 10.1186/s13104-019-4241-0
112. Shanafelt TD, Drake MT, Maurer MJ, Allmer C, Rabe KG, Slager SL, et al. Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood. (2011) 117:1492–8. doi: 10.1182/blood-2010-07-295683
113. Ding EL, Mehta S, Fawzi WW, Giovannucci EL. Interaction of estrogen therapy with calcium and vitamin D supplementation on colorectal cancer risk: reanalysis of Women's Health Initiative randomized trial. Int J Cancer. (2008) 122:1690–4. doi: 10.1002/ijc.23311
114. Antunac Golubic Z, Barsic I, Librenjak N, Plestina S. Vitamin D supplementation and survival in metastatic colorectal cancer. Nutr Cancer. (2018) 70:413–7. doi: 10.1080/01635581.2018.1445766
115. Brandstedt J, Almquist M, Manjer J, Malm J. Vitamin D, PTH, and calcium and the risk of prostate cancer: a prospective nested case-control study. Cancer Causes Control. (2012) 23:1377–85. doi: 10.1007/s10552-012-9948-3
116. Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. (2008) 100:1581–91. doi: 10.1093/jnci/djn360
117. Chen PT, Hsieh CC, Wu CT, Yen TC, Lin PY, Chen WC, et al. 1alpha,25-dihydroxyvitamin D3 inhibits esophageal squamous cell carcinoma progression by reducing IL6 signaling. Mol Cancer Ther. (2015) 14:1365–75. doi: 10.1158/1535-7163.MCT-14-0952
118. Barry EL, Peacock JL, Rees JR, Bostick RM, Robertson DJ, Bresalier RS, et al. Vitamin D receptor genotype, vitamin D3 supplementation, and risk of colorectal adenomas: a randomized clinical trial. JAMA Oncol. (2017) 3:628–35. doi: 10.1001/jamaoncol.2016.5917
119. Huerta S, Irwin RW, Heber D, Go VL, Koeffler HP, Uskokovic MR, et al. 1alpha,25-(OH)(2)-D(3) and its synthetic analogue decrease tumor load in the Apc(min) Mouse. Cancer Res. (2002) 62:741–6.
120. Otoshi T, Iwata H, Kitano M, Nishizawa Y, Morii H, Yano Y, et al. Inhibition of intestinal tumor development in rat multi-organ carcinogenesis and aberrant crypt foci in rat colon carcinogenesis by 22-oxa-calcitriol, a synthetic analogue of 1 alpha, 25-dihydroxyvitamin D3. Carcinogenesis. (1995) 16:2091–7. doi: 10.1093/carcin/16.9.2091
121. Chiang KC, Yeh CN, Hsu JT, Chen LW, Kuo SF, Sun CC, et al. MART-10, a novel vitamin D analog, inhibits head and neck squamous carcinoma cells growth through cell cycle arrest at G0/G1 with upregulation of p21 and p27 and downregulation of telomerase. J Steroid Biochem Mol Biol. (2013) 138:427–34. doi: 10.1016/j.jsbmb.2013.09.002
122. Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. (2005) 97:153–64. doi: 10.1016/j.jsbmb.2005.06.024
123. Zhou JY, Norman AW, Chen DL, Sun GW, Uskokovic M, Koeffler HP. 1,25-Dihydroxy-16-ene-23-yne-vitamin D3 prolongs survival time of leukemic mice. Proc Natl Acad Sci USA. (1990) 87:3929–32. doi: 10.1073/pnas.87.10.3929
124. Banach-Petrosky W, Ouyang X, Gao H, Nader K, Ji Y, Suh N, et al. Vitamin D inhibits the formation of prostatic intraepithelial neoplasia in Nkx3.1;Pten mutant mice. Clin Cancer Res. (2006) 12:5895–901. doi: 10.1158/1078-0432.CCR-06-1039
125. Whitlatch LW, Young MV, Schwartz GG, Flanagan JN, Burnstein KL, Lokeshwar BL, et al. 25-Hydroxyvitamin D-1alpha-hydroxylase activity is diminished in human prostate cancer cells and is enhanced by gene transfer. J Steroid Biochem Mol Biol. (2002) 81:135–40. doi: 10.1016/S0960-0760(02)00053-5
126. Hsu JY, Feldman D, McNeal JE, Peehl DM. Reduced 1alpha-hydroxylase activity in human prostate cancer cells correlates with decreased susceptibility to 25-hydroxyvitamin D3-induced growth inhibition. Cancer Res. (2001) 61:2852–6.
127. Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. (2014) 14:342–57. doi: 10.1038/nrc3691
128. Hoyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jaattela M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. (2005) 12:1297–309. doi: 10.1038/sj.cdd.4401651
129. Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. (2012) 441:61–76. doi: 10.1042/BJ20110744
130. Jiang F, Bao J, Li P, Nicosia SV, Bai W. Induction of ovarian cancer cell apoptosis by 1,25-dihydroxyvitamin D3 through the down-regulation of telomerase. J Biol Chem. (2004) 279:53213–21. doi: 10.1074/jbc.M410395200
131. Wang QM, Jones JB, Studzinski GP. Cyclin-dependent kinase inhibitor p27 as a mediator of the G1-S phase block induced by 1,25-dihydroxyvitamin D3 in HL60 cells. Cancer Res. (1996) 56:264–7.
132. Pendas-Franco N, Garcia JM, Pena C, Valle N, Palmer HG, Heinaniemi M, et al. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene. (2008) 27:4467–77. doi: 10.1038/onc.2008.88
133. Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. (2007) 6:1433–9. doi: 10.1158/1535-7163.MCT-06-0677
134. Iseki K, Tatsuta M, Uehara H, Iishi H, Yano H, Sakai N, et al. Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int J Cancer. (1999) 81:730–3. doi: 10.1002/(SICI)1097-0215(19990531)81:5<730::AID-IJC11>3.3.CO;2-H
135. Tavera-Mendoza LE, Westerling T, Libby E, Marusyk A, Cato L, Cassani R, et al. Vitamin D receptor regulates autophagy in the normal mammary gland and in luminal breast cancer cells. Proc Natl Acad Sci USA. (2017) 114:E2186–94. doi: 10.1073/pnas.1615015114
136. Bruns H, Buttner M, Fabri M, Mougiakakos D, Bittenbring JT, Hoffmann MH, et al. Vitamin D-dependent induction of cathelicidin in human macrophages results in cytotoxicity against high-grade B cell lymphoma. Sci Transl Med. (2015) 7:282ra47. doi: 10.1126/scitranslmed.aaa3230
137. Busch L, Mougiakakos D, Buttner-Herold M, Muller MJ, Volmer DA, Bach C, et al. Lenalidomide enhances MOR202-dependent macrophage-mediated effector functions via the vitamin D pathway. Leukemia. (2018) 32:2445–58. doi: 10.1038/s41375-018-0114-0
138. Flamann C, Busch L, Mackensen A, Bruns H. Combination of lenalidomide and vitamin D enhances MOR202-mediated cytotoxicity of macrophages: it takes three to tango. Oncotarget. (2019) 10:10–2. doi: 10.18632/oncotarget.26531
139. Bruns H, Bottcher M, Qorraj M, Fabri M, Jitschin S, Dindorf J, et al. CLL-cell-mediated MDSC induction by exosomal miR-155 transfer is disrupted by vitamin Leukemia D. (2017) 31:985–8. doi: 10.1038/leu.2016.378
140. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. (2001) 357:539–45. doi: 10.1016/S0140-6736(00)04046-0
141. Menkin V. Role of inflammation in carcinogenesis. Br Med J. (1960) 1:1585–94. doi: 10.1136/bmj.1.5186.1585
142. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. (2001) 48:526–35. doi: 10.1136/gut.48.4.526
143. Krishnan AV, Feldman D. Molecular pathways mediating the anti-inflammatory effects of calcitriol: implications for prostate cancer chemoprevention and treatment. Endocr Relat Cancer. (2010) 17:R19–38. doi: 10.1677/ERC-09-0139
144. Nonn L, Peng L, Feldman D, Peehl DM. Inhibition of p38 by vitamin D reduces interleukin-6 production in normal prostate cells via mitogen-activated protein kinase phosphatase 5: implications for prostate cancer prevention by vitamin D. Cancer Res. (2006) 66:4516–24. doi: 10.1158/0008-5472.CAN-05-3796
145. Wallace G, Jodele S, Myers KC, Dandoy CE, El-Bietar J, Nelson A, et al. Single ultra-high-dose cholecalciferol to prevent vitamin D deficiency in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2018) 24:1856–60. doi: 10.1016/j.bbmt.2018.05.019
146. Leyssens C, Verlinden L, Verstuyf A. The future of vitamin D analogs. Front Physiol. (2014) 5:122. doi: 10.3389/fphys.2014.00122
147. Alagbala AA, Johnson CS, Trump DL, Posner GH, Foster BA. Antitumor effects of two less-calcemic vitamin D analogs (Paricalcitol and QW-1624F2-2) in squamous cell carcinoma cells. Oncology. (2006) 70:483–92. doi: 10.1159/000098813
148. von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat Immunol. (2010) 11:344–9. doi: 10.1038/ni.1851
149. Bendix-Struve M, Bartels LE, Agnholt J, Dige A, Jorgensen SP, Dahlerup JF. Vitamin D3 treatment of Crohn's disease patients increases stimulated T cell IL-6 production and proliferation. Aliment Pharmacol Ther. (2010) 32:1364–72. doi: 10.1111/j.1365-2036.2010.04463.x
Keywords: vitamin D, GvH, GvL, immune balance, macrophages, T cells, infection
Citation: Flamann C, Peter K, Kreutz M and Bruns H (2019) Regulation of the Immune Balance During Allogeneic Hematopoietic Stem Cell Transplantation by Vitamin D. Front. Immunol. 10:2586. doi: 10.3389/fimmu.2019.02586
Received: 08 July 2019; Accepted: 18 October 2019;
Published: 05 November 2019.
Edited by:
Susu M. Zughaier, Qatar University, QatarReviewed by:
Ran Reshef, Columbia University, United StatesAntonio Pierini, University of Perugia, Italy
Copyright © 2019 Flamann, Peter, Kreutz and Bruns. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heiko Bruns, heiko.bruns@uk-erlangen.de
 Cindy Flamann
Cindy Flamann Katrin Peter
Katrin Peter Marina Kreutz
Marina Kreutz Heiko Bruns
Heiko Bruns