Integrated ecosystem assessment around islands of the tropical South Mid-Atlantic Ridge
- 1Fisheries Ecosystems Laboratory (LabPesq), Oceanographic Institute, University of São Paulo, São Paulo, Brazil
- 2Marine Macroecology and Biogeography Laboratory, Universidade Federal de Santa Catarina, Florianopolis, Brazil
- 3Oceanographic Institute, University of São Paulo, São Paulo, Brazil
- 4Universidade Federal do Oeste do Pará, Oriximiná, Brazil
- 5Reef Systems Ecology and Conservation Lab, Universidade Federal Fluminense, Niterói, Brazil
- 6School of Biological and Marine Sciences, Plymouth University, Plymouth, United Kingdom
The South Mid Atlantic Ridge comprises three main oceanic islands in the equatorial and tropical portions of the Atlantic Ocean. These islands are isolated from each other and equidistant from both the continental margins of South America and Africa, sharing common patterns but with different types of human use and pressures. Moreover, the areas beyond national jurisdiction between those islands are visited and exploited by distant fishing fleets and include large areas of shipping activity for commodities. Here, a pioneering integrated ecosystem assessment (IEA) process is constructed for the region among Saint Peter and Saint Paul’s Archipelago (Brazil), Saint Helena Island and Ascension Island (UK overseas territories). For that, we used a qualitative assessment of risks arising from anthropogenic activities, representing a novel contribution to the field. The Options for Delivering Ecosystem-Based Marine Management (ODEMM) approach was applied to trace sector–pressure–component pathways. A ‘linkage framework’ was outlined including pressures affecting each ecosystem component, and supported a process of knowledge attributions that scored the impact risks. All results were validated with regional stakeholders through workshops, including local and international management bodies, non-governmental organizations (NGOs) and scientists. The approach focused on a significant area among encompassing the open ocean, shallow and deep-sea biomes, analyzing the main sectors and pressures affecting the ecological components. Our results identified 14 sectors and 16 key pressures associated with 23 ecosystem components, totaling 780 impact chains. Fishing, shipping, wastewater, and tourism/recreation appeared as the top impacting sectors. Fishing and shipping were the most connected with ecosystem components links. Litter, species extraction, contaminants, and bycatch were the pressures that had the highest risk of impact values. Lastly, demersal and pelagic fish and pelagic and demersal elasmobranchs were the groups with the highest risk related to overall impacts, which were supported by local and regional evidence from long term monitoring programs and local studies. Our study demonstrated that these seemingly pristine islands and oceanic waters are already experiencing human impacts that should be addressed by local both conservation measures and international agreements. We also highlight the pressures that should be prioritized for better monitoring and policy, as well as those linkage components that have been less investigated.
1 Introduction
Anthropogenic activities in the oceans are intricately linked with the state of the ecological systems and the ecosystem services they provide. Understanding these links presents an immense challenge for management and conservation initiatives, and requires a multidisciplinary approach. This challenge is even greater in areas beyond national jurisdiction (ABNJ), where users often may be in different jurisdictions regulated by different political systems, and with different value arrangements and socio-economic interests (Rogers et al., 2014; Popova et al., 2019).
Ecosystem-based management (EBM) is widely supported as a holistic approach for improved environmental management, because it recognizes the ecological components and socio-economic systems and their interrelationships (Piet et al., 2015; Cormier et al., 2017; Piet et al., 2020). EBM considers humans as a part of the ecosystem, and the interaction among ecosystem components and management sectors is prioritized (Levin et al., 2009; AORA, 2019; O’Higgins et al., 2020). Although the EBM concept and approach are quite well defined, its effective application is not, particularly when there is a lack of scientific knowledge and data (Möllmann et al., 2014; ICES, 2021). The principle of EBM is that all individual ecosystem components are linked to other components within a coupled socio-ecological system that should be described as a first step. Thus, an Integrated Ecosystem Assessment (IEA) takes into account the fundamental principles of EBM, however, for practical effectiveness, the IEA requires a comprehensive risk analysis and is implemented collaboratively, involving scientists, managers, and stakeholders, with the aim of balancing trade-offs and determining what is more likely to achieve regarding their goals. This iterative approach with stakeholders provides a stronger foundation for supporting ecosystem-based management.
In this sense, an IEA offers a holistic perspective combining socio-economic and biological aspects that maintain ecosystem dynamics (Levin et al., 2014). Studies based on integrated ecosystem approaches are necessary for a more connected understanding of the whole ecosystem. In fact, to make EBM easier to be implemented by management authorities, Levin et al. (2009) proposed the IEAs, that is set as “a framework for organizing science to inform decisions in marine EBM at multiple scales and across sectors to achieve multiple simultaneous ecosystem objectives’’. This framework outlines a five-step process: scoping, indicator development, risk analysis, management strategy evaluation and ecosystem assessment (Levin et al., 2009; Levin et al., 2014; Samhouri et al., 2014).
The IEA approach is flexible to use different tools and can be adapted to regional needs. A key component of an IEA is the evaluation of potential risks of human activities and natural perturbations on ecosystems (Levin et al., 2009; Hobday et al., 2011; Levin et al., 2014; Möllmann et al., 2014; Pedreschi et al., 2019). Those risk assessments may use quantitative data (e.g., Fletcher, 2005), qualitative data (e.g., Options for Delivering Ecosystem-based Marine Management-ODEMM, Fletcher et al., 2010; Breen et al., 2012; Robinson et al., 2014) which support broad assessments best applied and interpreted as a screening tool (Knights et al., 2015), or a combination of the two (e.g., Samhouri and Levin, 2012; Fletcher et al., 2014). In this study, we used the ODEMM (Options for Delivering Ecosystem-based Marine Management) approach (Robinson et al., 2014) to identify the greatest impact risks on marine ecosystem components. This tool was chosen because it is based on a clear structure that can be replicable and used for comparative analyses, and can be applied in data-poor situations (Robinson et al., 2014) which was the case here. ODEMM is a method that advises the scoping phase of an IEA, also providing a scoring schedule.
The equatorial and tropical region of the South Mid Atlantic Ridge features three oceanic islands and their respective Exclusive Economic Zones (EEZ): Saint Peter and Saint Paul’s Archipelago (St Paul’s Rocks), Ascension and St Helena Islands (Figure 1). The island´s EEZs and the ABNJ between them support populations of late-maturing and long-lived species (Shackeroff et al., 2009); they are critical habitats for species with long migratory pathways such as sharks, sea turtles and whales (Edwards, 1990; Burns et al., 2020; IUCN, 2022). These regions host a diversity of uses, including navigation, fishing, and scientific research, with energy resources and mineral extraction being considered as emerging issues (IUCN, 2022). Due to the need for a huge range of data and/or expert knowledge, data-limited locations, such as the southern Mid-Atlantic Ridge area, are rarely assessed through an integrated approach. Fortunately, there are two recent studies that provide potential approaches that can be applied in such data-limited situations. Mynott et al. (2021) proposed a standardized approach to risk assessment for sand extraction being adapted to isolated sites with limited data, as the case for Saint Helena Island. Similarly, Hardman et al. (2022) provide an example of a study of integrated marine management in small islands, focusing on a balance between conservation and socioeconomic priorities.
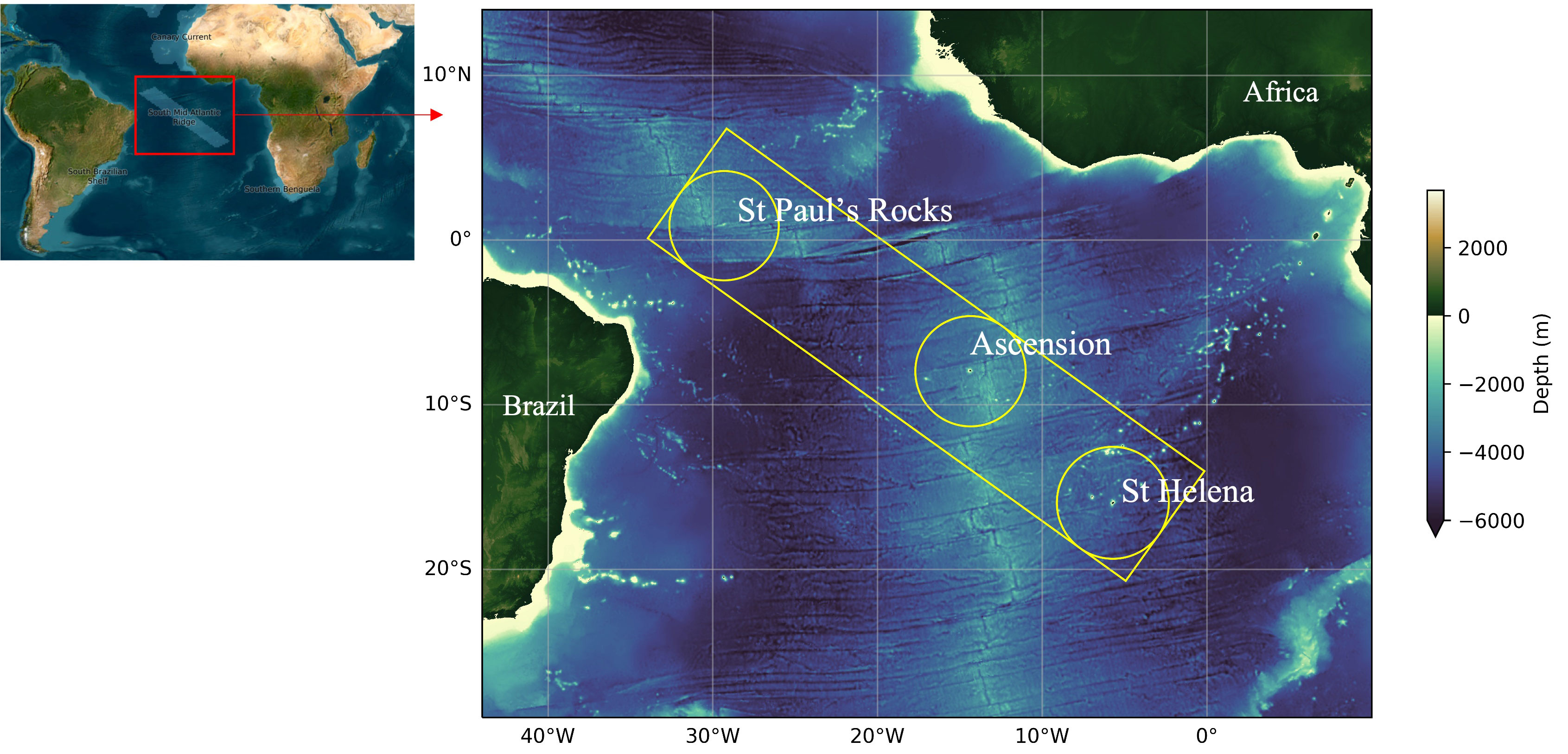
Figure 1 South Mid-Atlantic Ridge study area (yellow polygon). Circles indicate the EEZ of St Paul’s Rocks, Ascension Islands and St Helena.
This study undertakes ODEMM approach in a large area from the Saint Peter and Saint Paul’s Archipelago to Saint Helena Island, including Ascension Island encompassing open ocean, shallow and deep-sea biomes. Here, we build on (i) a linkage framework (the impacts chains) to identify potential pressures between multiple sectors in the marine environment, and (ii) an Impact Risk (IR) score framework to rank the principal sectors, pressures and ecological components with high potential risk to the ecosystems. Lastly, we highlight components and research gaps which should be priorities for future management and conservation efforts.
2 Materials and methods
2.1 Description of study area
Our study area includes the three isolated oceanic islands including the South Mid-Atlantic Ridge islands, St Paul’s Rocks (i.e., St. Peter and St. Paul Archipelago - SPSPA), Ascension and St Helena, located in the tropical and equatorial bands of the South Atlantic Ocean (Figure 1). These islands are topographic manifestations of the Mid-Atlantic Ridge that extends in the meridional direction in the Mid-Atlantic basin. The three islands have many aspects in common, including that they are small, very isolated, equidistant from South America and Africa, and located within an area of relatively warm and oligotrophic oceanic waters (Edwards and Lubbock, 1983a; Edwards and Lubbock, 1983b; Edwards, 1990). They share many species, including some endemics and sister-species that are exclusive to the three islands (Edwards and Lubbock, 1983a; Joyeux et al., 2001; Floeter et al., 2008; Wirtz et al., 2014; Brown et al., 2019; Pinheiro et al., 2020). Marine Protected Areas (MPAs) in Saint Helena and Ascension were designated in 2016 and 2019, respectively, covering 100% of both EEZs (Saint Helena Government, 2016; Ascension Island Government, 2021). This initiative makes possible to protect different habitats and the natural processes that support them, as well as respecting the cultural significance of the ocean to people living on such islands. St Helena’s MPA is considered a protected area of sustainable use of natural resources. For example, some fishing methods are prohibited within St Helena’s MPA such as purse seining, longlining and greensticking, but handlines, pole-and-line, pots, and spear guns are permitted on some local rocky shores. St Helena, however, still lacks no-take areas in the shallow waters around the island (St Helena Government, 2022). Around Ascension Island, despite a local MPA covering the EEZ, no-take areas are also absent. The MPA that comprises the SPSPA EEZ includes two categories of protected areas, a multiple-use area, and a smaller no-take area (Giglio et al., 2018). Human populations living on the islands range from four people inhabiting SPSPA to around 4,500 in St Helena.
Thus, the study area of this IEA covers the three islands’ EEZs (economic exclusive zones of both Brazil and UK) and the ABNJ between the islands, which concentrate high intensity. Both the distribution of pelagic fish and its fisheries have important hotspots close to the boundaries of the islands EEZs (Global Fishing Watch, 2021; ICCAT, 2022), that does not necessarily follow the Mid-Atlantic Ridge. For example, the region south of SPSPA and west of Ascension Island is especially targeted by the international tuna fishing industry (Muench et al., 2022). Hence, for these resources, the ridge seems to be less important than other oceanographic factors, e.g. currents or zones of higher primary productivity. For this reason, the study area is not only focusing on the ridge, but also includes the regions between the islands. The oceanographic characteristics, biogeographical species connectivity among the three islands, and the spatial distribution of the fishing fleets justifies the need of an ecosystem assessment of this particular region of the South Atlantic Ocean.
Pelagic fishing in the study region are has been taking place since 1956 and is carried out by boats under different flags, such as Japan, Taiwan, Korea, Spain, China, Portugal, among others (Global Fishing Watch, 2021). The high seas bottom fisheries of the southeast Atlantic are recent and still underdeveloped. A variety of species have been exploited on slopes and also around the island EEZs of the British Overseas Territory (FAO, 2020). The study area includes areas of moderate and high shipping intensity. The highest intensity is concentrated in the northern part of the region around Saint Peter and Saint Paul Archipelago1.
A large-scale circulation feature in this basin is the subtropical gyre, with the Brazil Current at its western boundary, the South Atlantic Current at the southern limit, and the Benguela Current and the South Equatorial Currents (SEC) at the eastern and the northern limit of the gyre (Stramma and England, 1999). SPSPA is located in the equatorial region under the influence of a complex system of zonal currents (the Northern (NSEC), the Equatorial (ESEC), the Central (CSEC) and the Southern (SSEC) branches of the Equatorial Currents) and undercurrents (Equatorial (EUC) and the South Equatorial Undercurrents (SEUC) (Talley et al., 2011; Luko et al., 2021). Ascension and St Helena Islands lay along the influence of the northward extension of the Benguela Current and the South Equatorial Current that flows south-westward. The South Atlantic Ocean has a unique role in the global meridional heat fluxes as it is the only basin transporting heat equatorward. The tropical and subtropical regions of the South Atlantic cover the path of the return flow of the global scale circulation known as the Atlantic Meridional Overturning Circulation (AMOC). It has been suggested that the influence of AMOC variations impact Atlantic sea surface temperatures (SST) (Duchez et al., 2016), and the important changes that are taking place in the evolving upper ocean circulation of the South Atlantic Ocean are likely to have consequent impacts on weather and the global climate (Marcello et al., 2018). The regions of high pelagic fishing intensity (the region south of SPSPA and west of Ascension Island) are under influence of the South Equatorial Countercurrent and the CSEC and exhibit relatively high primary production rates between July and September.
2.2 Summary semi-quantitative assessment approach
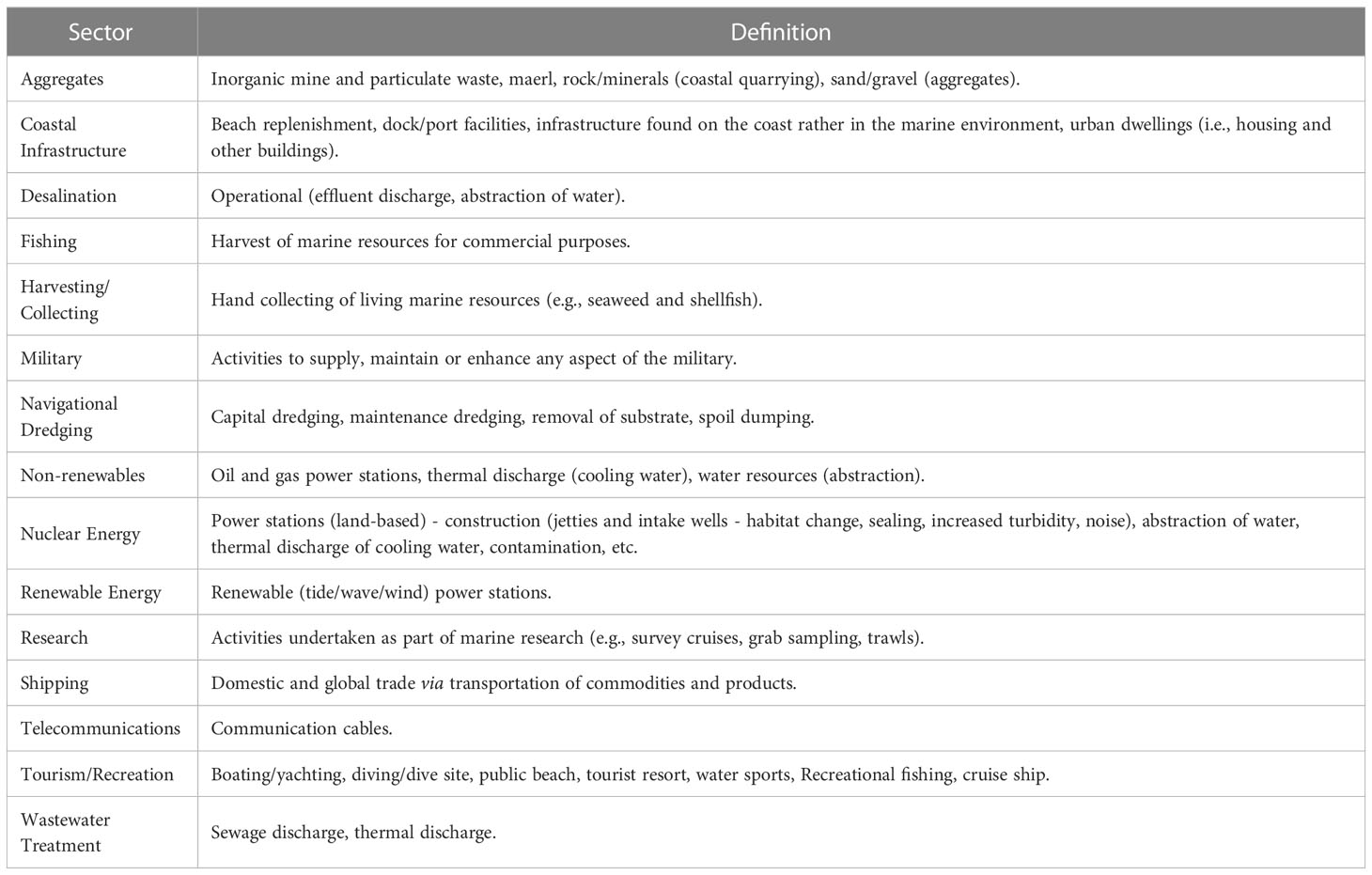
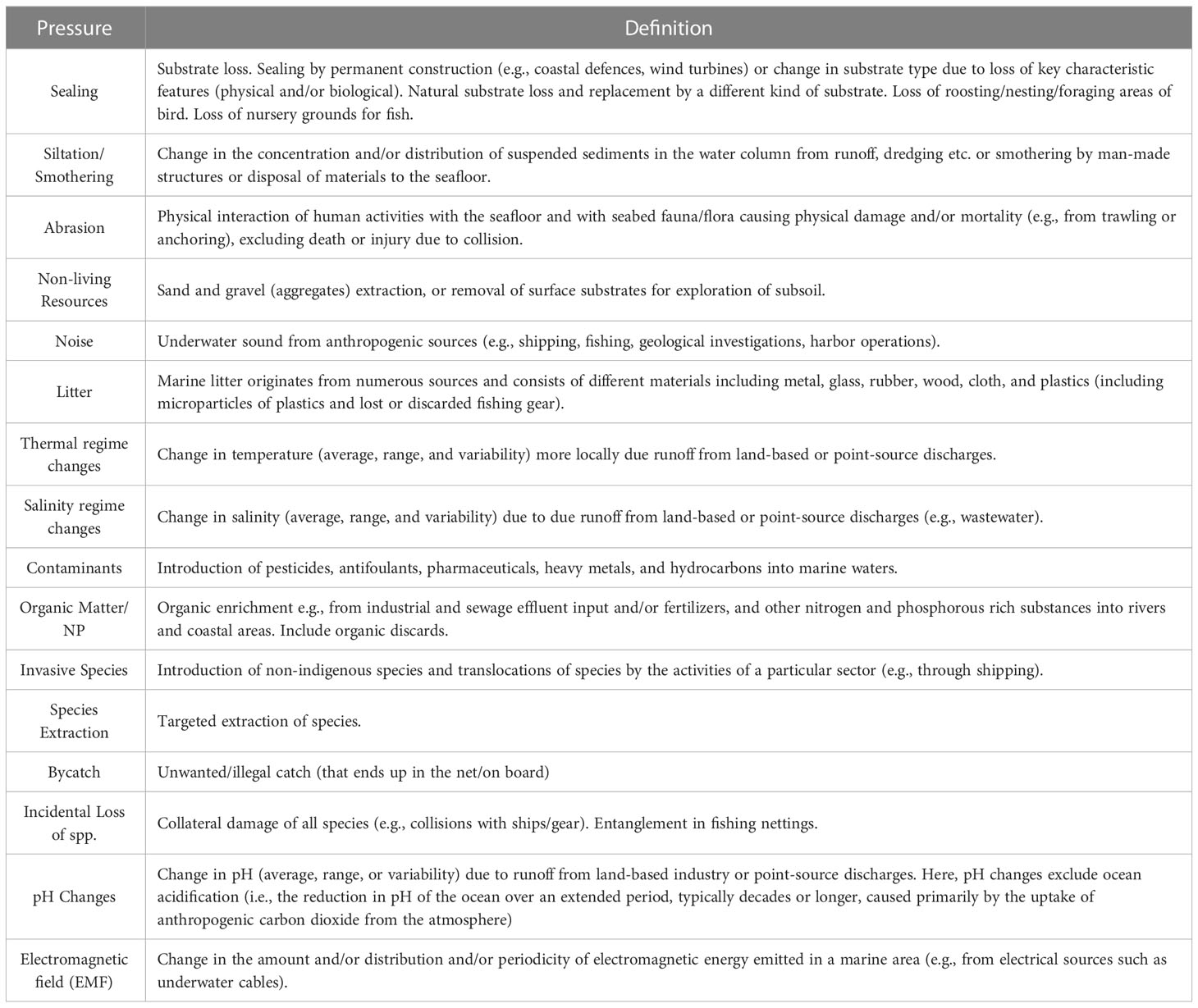
The first step consisted of identifying, in the study area context, the sectors (Table 1) and their pressures (Table 2) that affect each ecological component (EcoCom) to build a ‘linkage framework’ matrix (Robinson and Culhane, 2020). EcoCom consists of species groups or habitats, where habitat types are loosely based on the European Nature Information System (EUNIS) (Figure 2). The selection of matrix elements was adapted from previous studies, such as Pedreschi et al. (2019) and Robinson et al. (2014). Adaptations consisted, for example, on the removal of some sectors that were not representative within the area, such as land-based industry, agriculture, and aquaculture. Using the linkage matrix it was possible to establish the connections (impact-chain) between the elements, then to extract all sectors/pressures combinations that can interact with any one ecological component (Robinson et al., 2013): for example, Fishery [sector] – Litter [pressure produced by Fisheries] – Seabirds [ecological component which was impacted by Litter from Fishery sector]. This exercise was carried out within an expert team, including scientific institutions and Ascension and St Helena state agencies, with support of institutionally-held data, and from their knowledge. The methodology applied considers only the current and direct effects of the sector–pressures on ecological components, although we recognize that indirect interactions can also play important roles in the ecological processes.
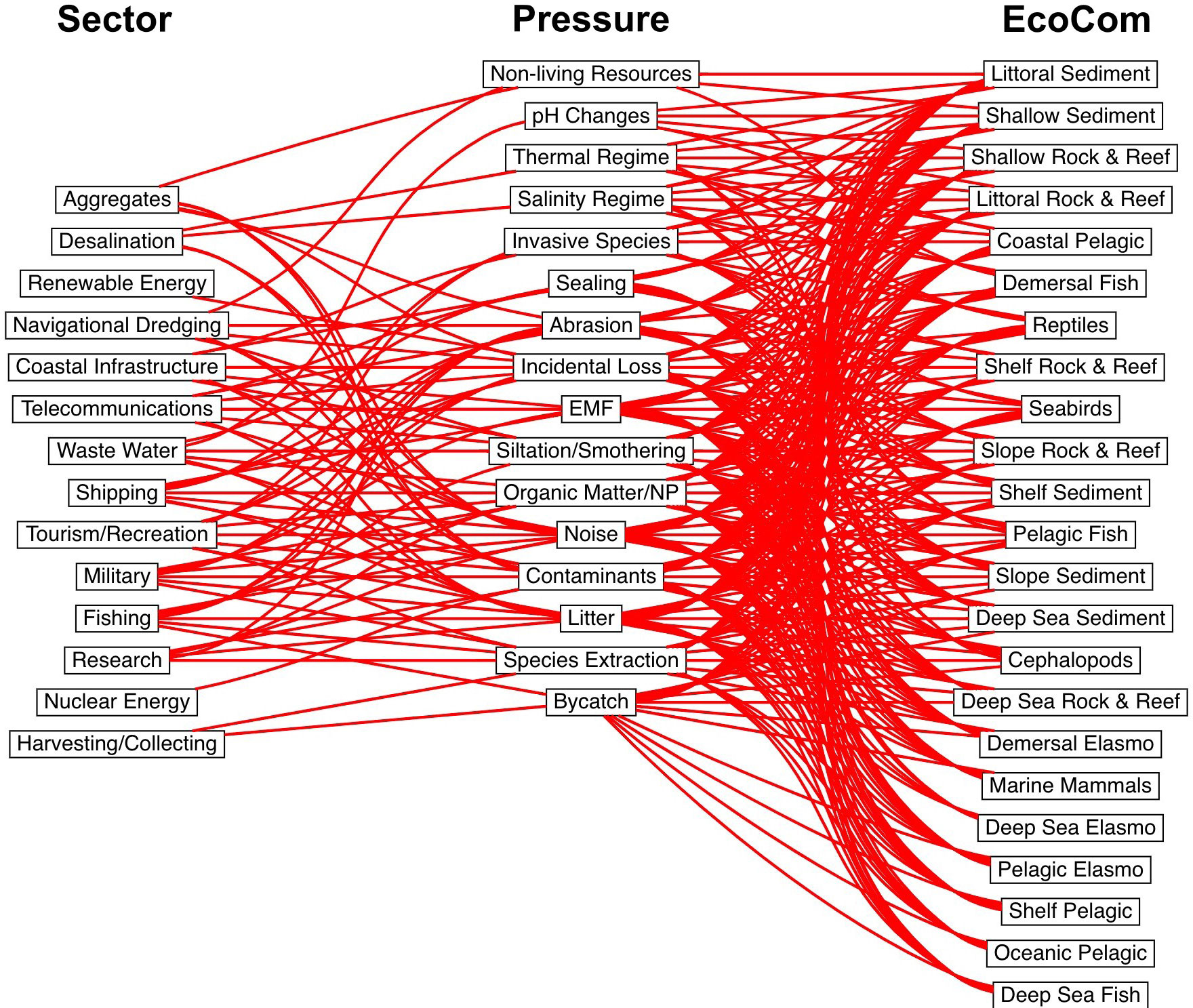
Figure 2 Horrendogram, illustrating the complexity of the linkage framework. All sectors, pressures and ecological components assessed in the case study area are indicated. Red lines indicate connections. EMF = Electromagnetic field. Elasmo = elasmobranchs. Interactive version can be found at https://ariccir.shinyapps.io/maio_2022/.
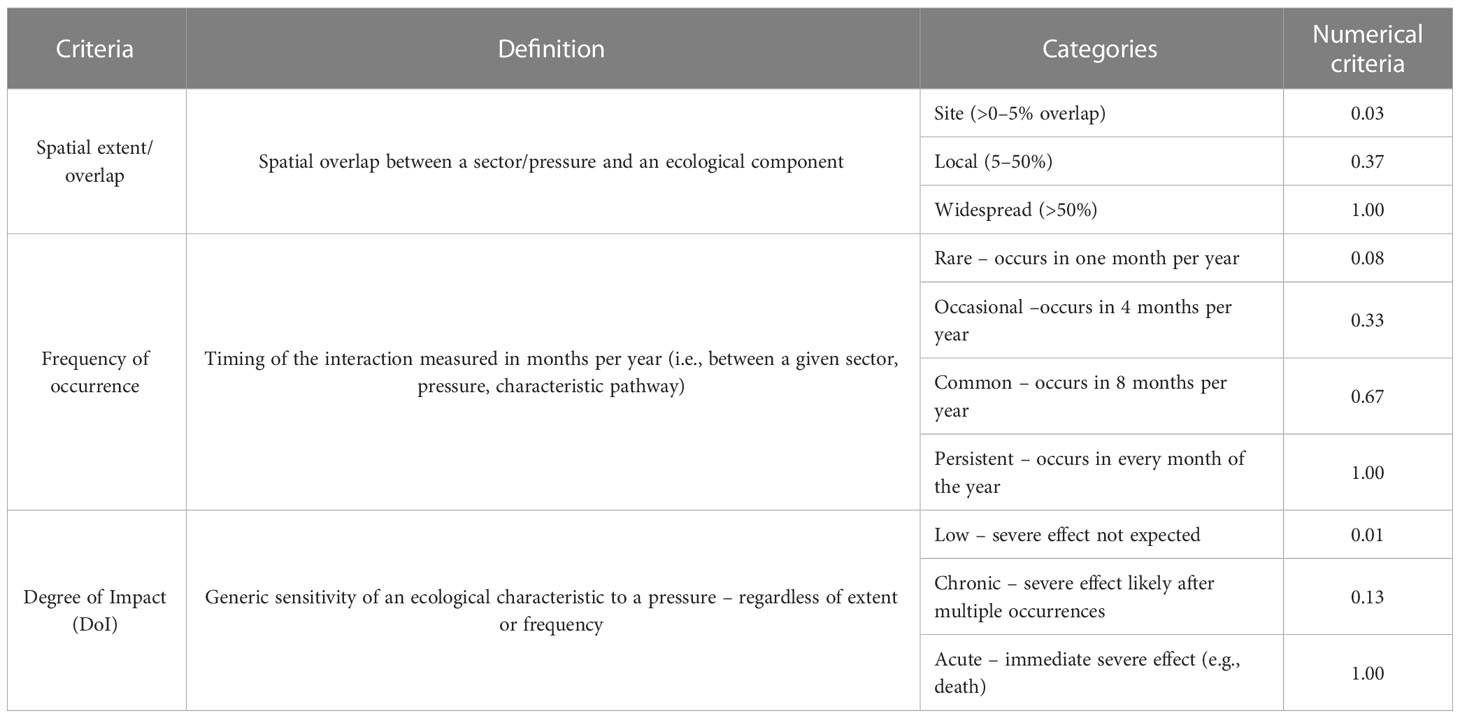
As a second step, successive meetings of a multi-disciplinary team of experts (expert judgment), such as specialists in coral reef and marine ecology, fisheries, oceanography, biogeography, ecotoxicology, and bioenergetic, were carried out to assign scores to each impact-chain, set in the linkage framework matrix. An extensive review on peer-reviewed and gray literature (reports, thesis, and conference abstracts) outlined in detail in the Supplementary Material 1 was used to elucidate connections and scores. For those connections with no bibliographic support, expert judgment was considered. Firstly, three criteria were used to assess qualitatively each impact chain (Table 3). These criteria are: (1) Spatial extent (overlap), and (2) Frequency of occurrence (temporal) to describe the exposure of the ecological component to a sector–pressure combination; and (3) Degree of impact (DoI) to describe the severity of interactions (Robinson et al., 2013 and Robinson et al., 2014). Although the ODEMM approach considers Resilience (recovery time) and Persistence criteria, neither were as yet assessed in this study, and will be considered as next steps. Then, each qualitative assessment criteria was converted into numerical criteria scores (Table 3) and were combined to produce the ‘Impact Risk’ score (Knights et al., 2015), following equation:
where: IR is the Impact Risk, Se is the Spatial extent, Fo is the Frequency of occurrence and DoI is the Degree of impact.
The IR scores, indicating the associated risk, were log transformed for better visual comparison between results, in which the greater the IR score, the greater the threat component. IR scores from multiple impact chains can be aggregated using different methods (Piet et al., 2017). Here, impact chains firstly were aggregated in three different groups: sectors, pressures and ecological components. Thereafter, the “top risk” (IR ranks) was determined by the sum and the average of impact risk scores of all impact chains separately by group. Both the sum and the average were used in the ranking process. Both descriptive statistics (sum and average) are influenced by the number of impact chains present and were used in the ranking process to avoid the methodological influence and bias, although ‘summation’ is less sensitive to such fluctuations (Piet et al., 2017; Pedreschi et al., 2019).
Another indicator used was ‘Proportional Connectance’ values, which is calculated as the number of impact chains (links) associated with each sector/pressure type/ecological components divided by the total number of impact chains in the ecosystem area expressed as a percentage. This indicator does not provide any indication of intensity, nor indication of which pathways may be the most critical, but how the assessed impact chains are connected (Pedreschi et al., 2019). Moreover, the Proportional Connectance is an additional index which provides a better view of the number of pressures that act on the greatest number of ecological characteristics (Pedreschi et al., 2019). Analyses were adapted from Pedreschi et al. (2019) for this study’s purposes and are available at https://github.com/gandrat/ODEMM.git.
Finally, to validate the semi-quantitative risk assessment, a workshop was carried out where the preliminary results were discussed with regional stakeholders representing several international and local institutions. Regional fisheries management organizations covering the broad region were invited, as well as local environmental management representatives from the 3 governments, international agencies and non-governmental organizations (NGOs) and regional experts from different academic institutions of Brazil and UK. A total of 8 marine experts with projects in some of the islands joined the discussion with 2 representatives of Brazil in the Intergovernamental Oceanographic Commission (IOC) and International Studies Association (ISA) (from the Navy), SEAFO, CECAF/FAO and IUCN-CEM representatives, 2 participants from industry and consulting groups, 2 participants from ICMBio (Brazilian Ministry of the Environment), 2 from NGOs, and 2 participants from St Helena and Ascension, in addition to the 8 project team members. Participants were then split into three groups according to their expertise in two separate rounds, first to discuss pressures and second to discuss ecological components. The groups were, round one: 1) litter and noise; 2) organic matter and contaminants; and 3) bycatch and species extraction. Round two: 1) pelagic resources; 2) sea bottom, coral reef, and demersal fish; and 3) cephalopods, seabirds, reptiles, and marine mammals. Overall, stakeholders agreed on the main results of the assessment, and furthermore shared valuable insights that were considered in the construction of the final results presented here.
3 Results
3.1 The ecosystem linkage framework
In total, 14 sectors (Table 4) and 16 pressures (Table 5) were identified as potentially responsible for harmful effects on each of the 23 ecological components (Table 6) across the study ecosystem. Thus, of 5,152 potential impact chains (i.e., all possible combinations between sectors, pressures and ecosystem components), the number of pathways (impact chains) identified in the ecosystem was 780 (Figure 2).
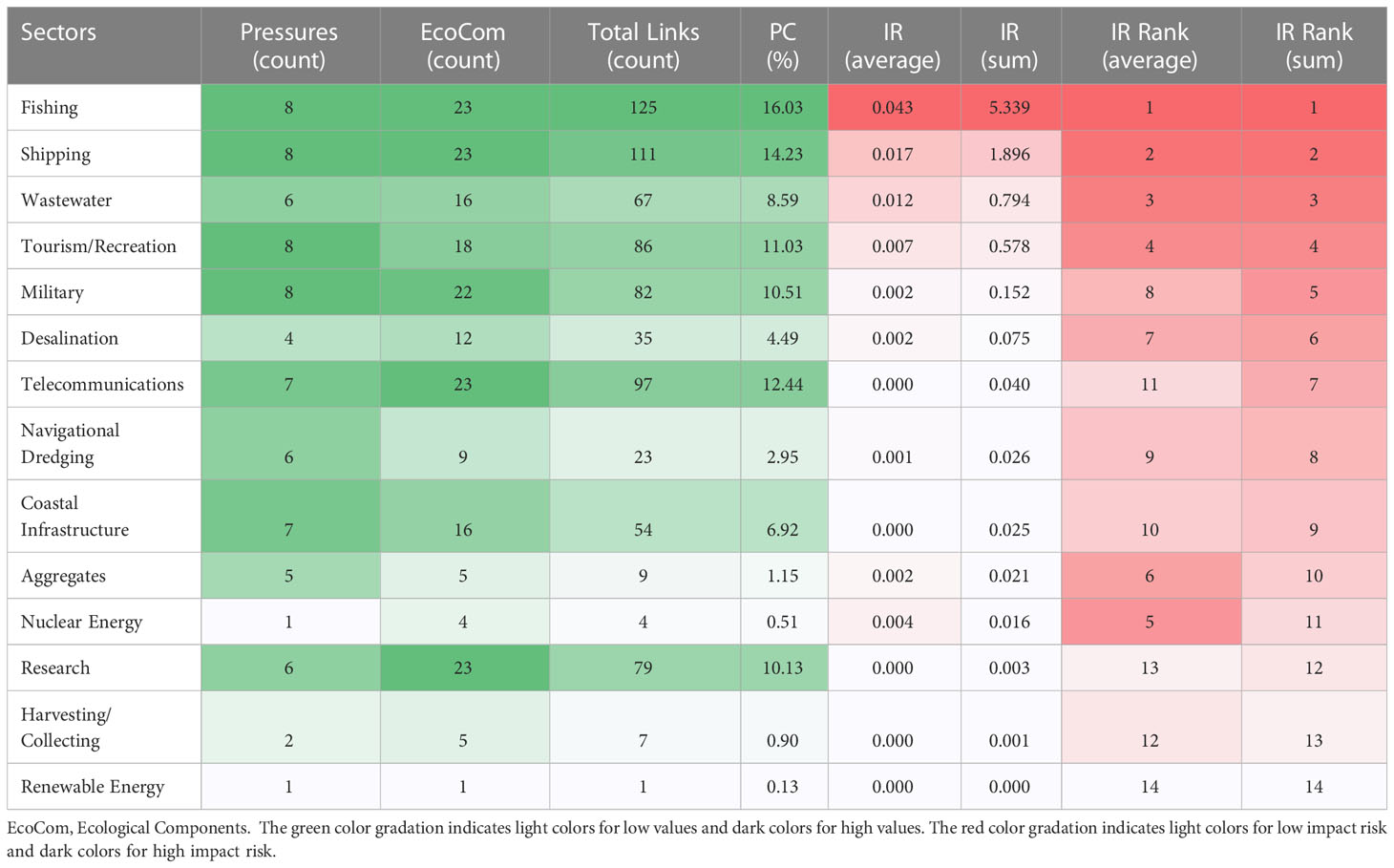
Table 4 Rankings of descriptors identified by the summed and average impact risk (IR) and their proportional connectance (PC), and sum and average risk scores for Sectors.
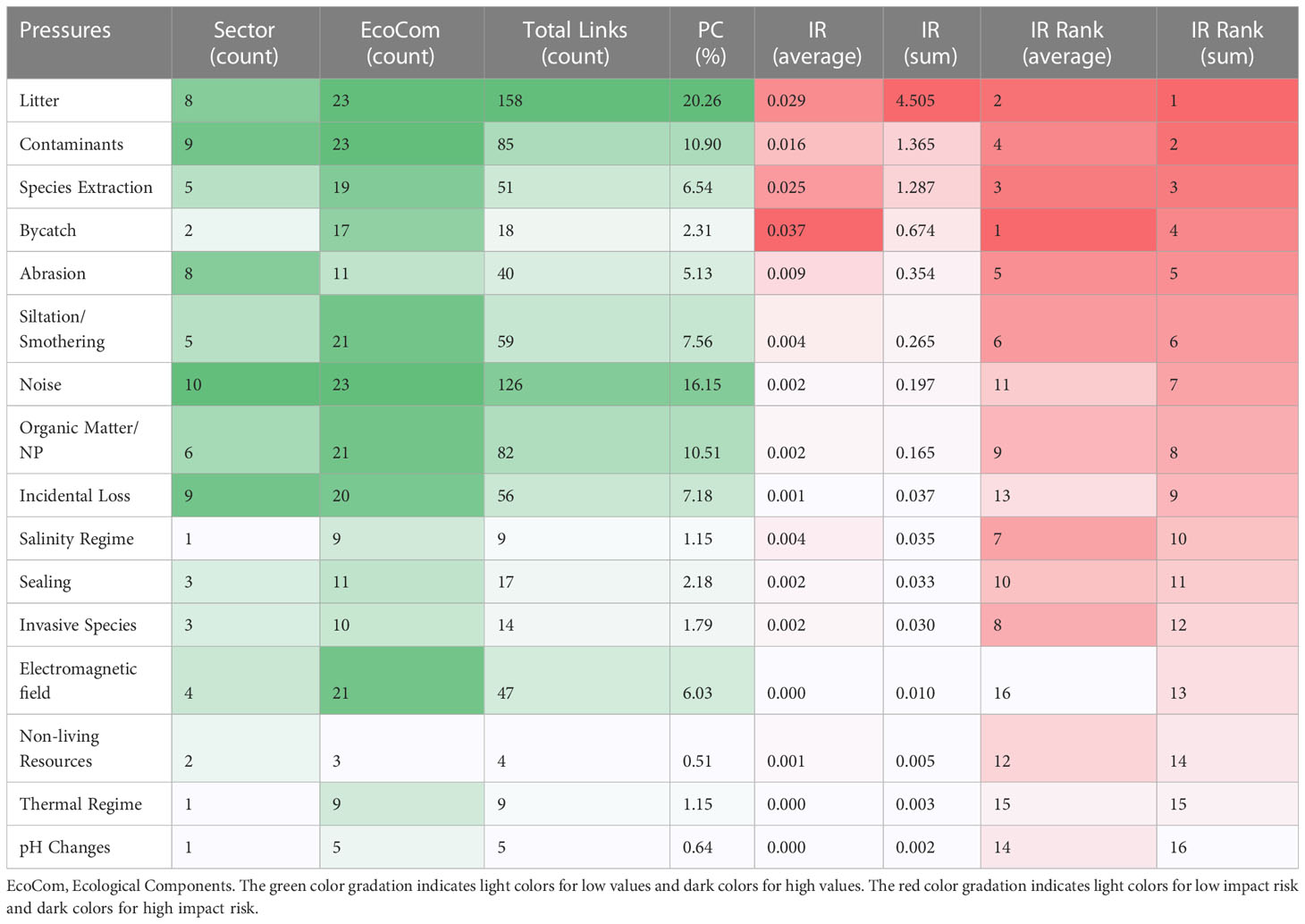
Table 5 Rankings of descriptors identified by the summed and average impact risk (IR) and their proportional connectance (PC), and sum and average risk scores for Pressures.
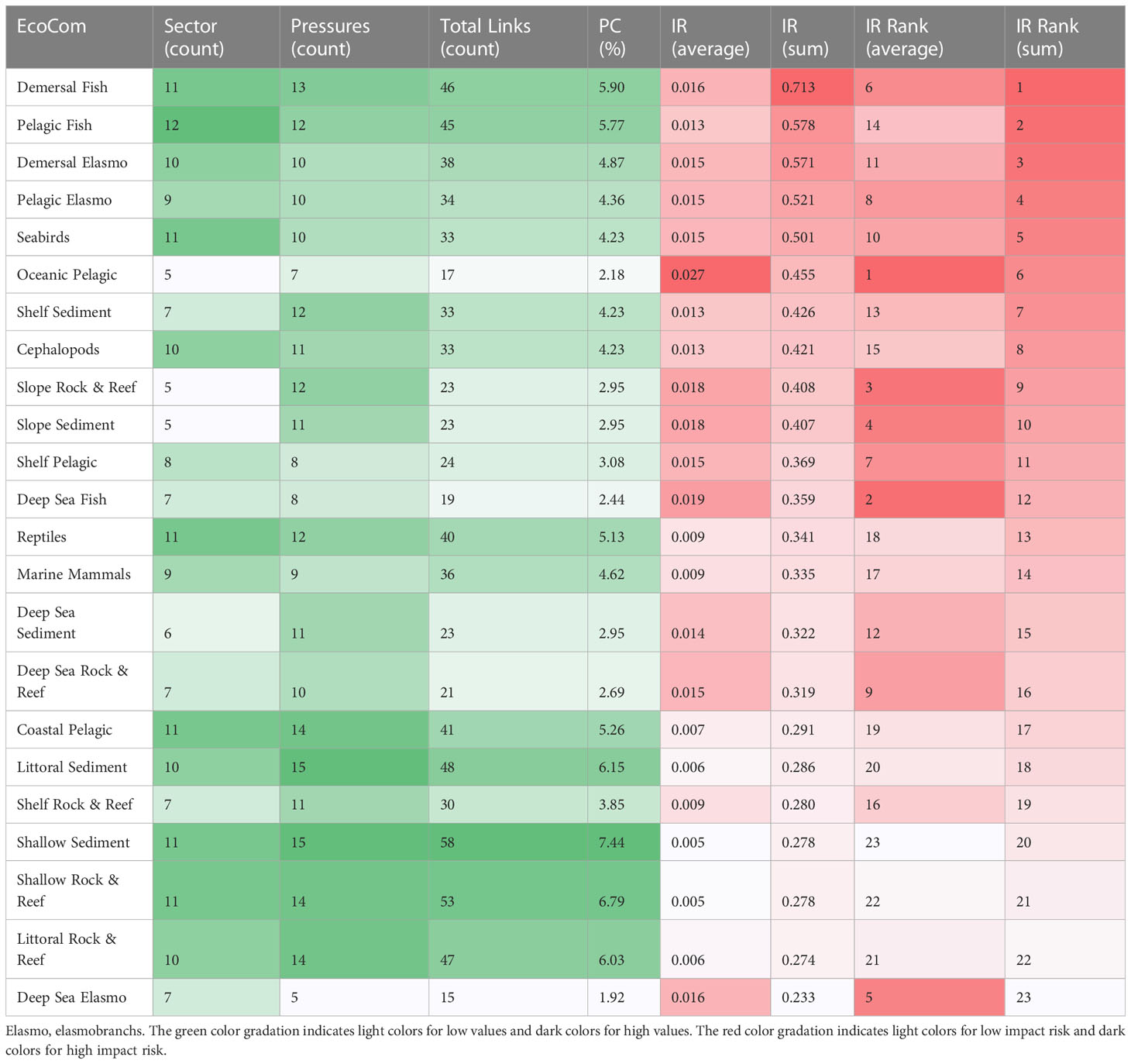
Table 6 Rankings of descriptors identified by the summed and average impact risk (IR) and their proportional connectance (PC), and sum and average risk scores for Ecological components (EcoCom).
3.2 Connectance and impact risks of sectors, pressures and ecological components
A detailed overview of individual scoring of each combination of sector–pressure –ecosystem components (assessment results of ‘proportional connectance’, ‘average impact risk’ and ‘sum impact risk’ by sector, pressure and ecosystem component) is presented in Tables 4–6. Fishing, shipping, telecommunications, and tourism/recreation were the sectors with the highest connectance, considering the proportion of linkages associated with each sector–pressure–ecological components. Therefore, fishing, shipping, wastewater and tourism/recreation demonstrated highest risk, considering the sum and average of impact risks (Table 4). Moreover, the sectors posing the greatest risk, indicated many widespread and frequent impact chains with severe consequences (Figures S1-S3 in Supplementary Material 2).
Regarding the pressures that have more influence on the study area, litter, contaminants, species extraction, bycatch and abrasion were considered the top five highest impact risks, although ranked differently depending on the sum and average of IR (Table 5).
Finally, concerning ecological components, shallow (<50 m depth) and littoral habitats (including benthic communities) have higher proportional connectance but a low score and average impact risk (Table 6). Contrary to what was observed in the sectors and pressures, a low level of consistency was found between the top five ecological components identified using the sum and average IR. The sum of IR scores indicates only marine organisms as being the groups with the highest impact risks, including demersal and pelagic fish, pelagic and demersal elasmobranchs, and seabirds (Table 6). On the other hand, habitat types and deep marine organisms, such as oceanic pelagic, deep-sea fish, slope rock and reef, slope sediment and deep sea elasmobranchs, were ranked higher using average IR, indicating that the choice of descriptive statistic method (sum or average) does matter.
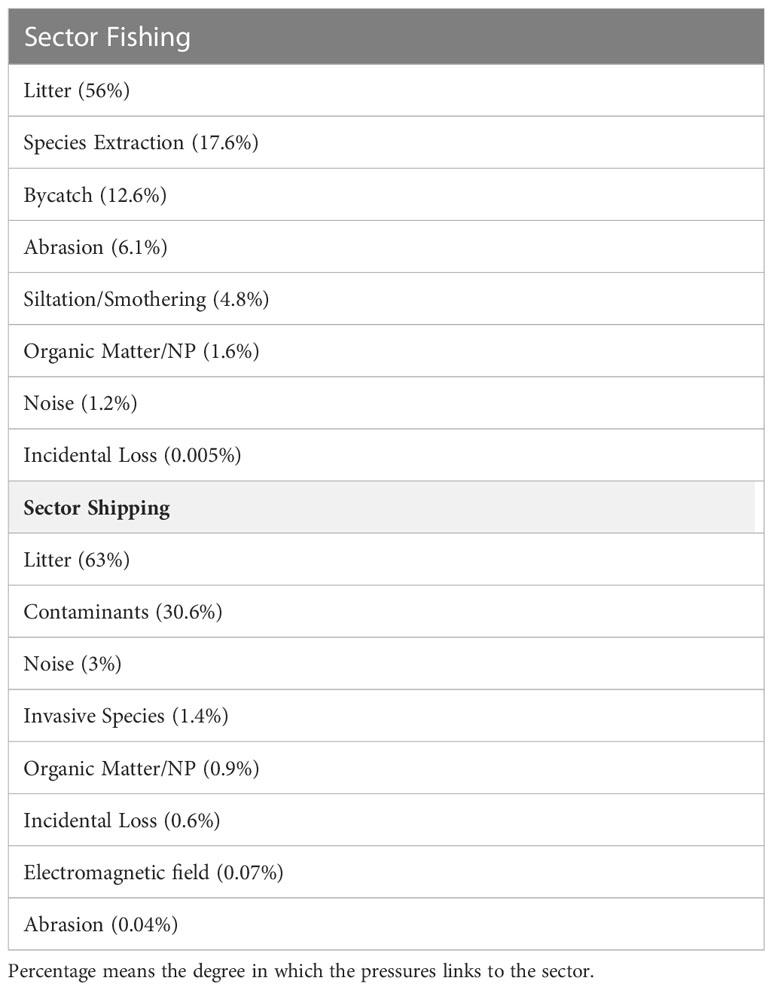
The most impactful sectors (fishing, shipping, wastewater and tourism/recreation) (Figure 3), further explain the connectances and impact risk score, regarding the pressures caused, as well as species and habitats at risk. These four sectors together contribute 96% to the overall sum IR (Table 4) and were related to 81% of the pressures identified in the area, which affect the 23 (100%) ecosystem components assessed. deep-sea species and habitats were associated with small risks of impact from these sectors. Fishing and shipping contribute more than 80% to the total IR (Table 4) and the principal source of pressure found was litter (Figure 3 and Table 7).
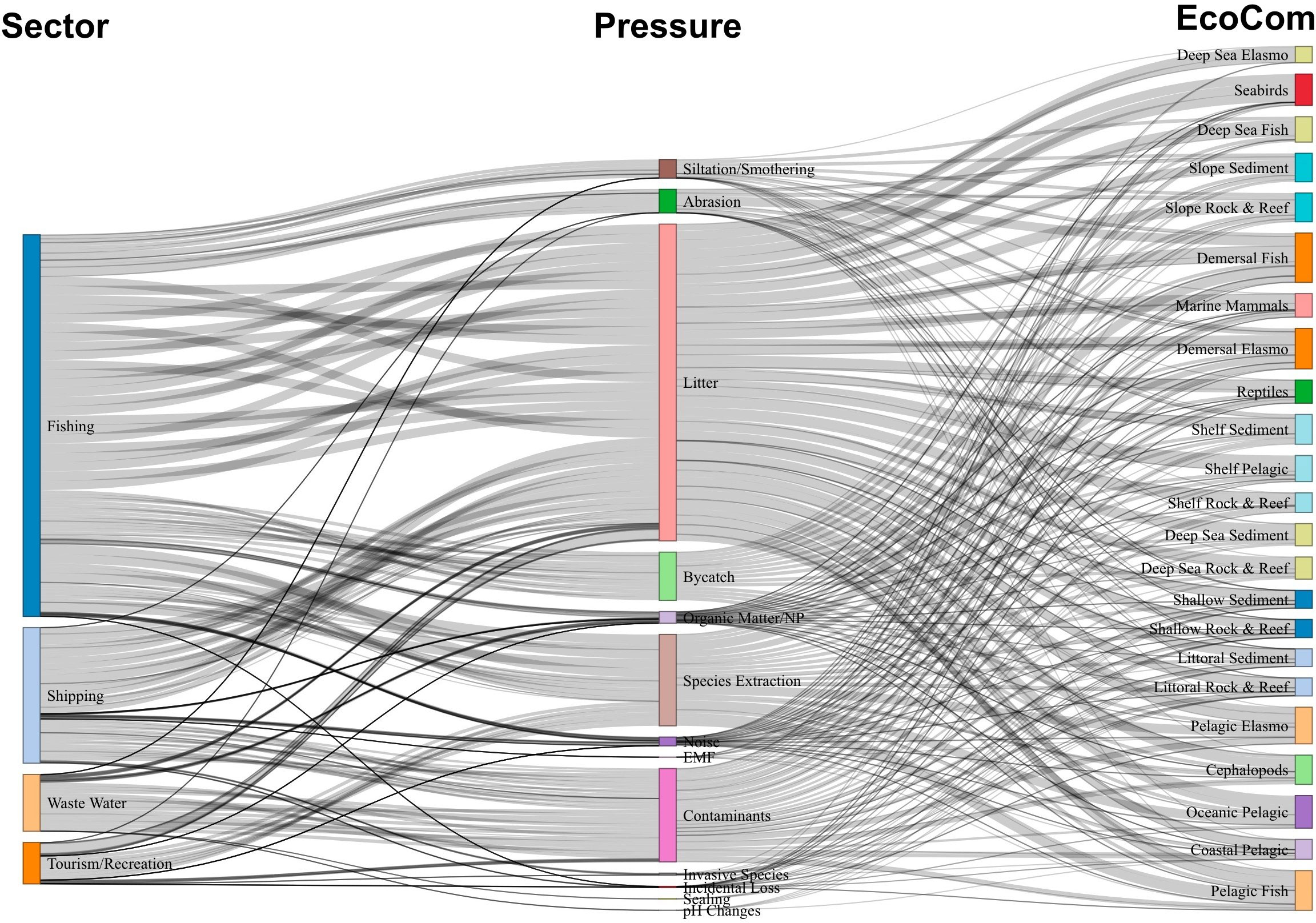
Figure 3 Sankey diagram of pathways among principal sectors (left), the pressures (middle) and the ecological components (EcoCom) of impacts (right). The width of lines represents the sum impact risk score (product of overlap, frequency, and degree of impact). EMF, electromagnetic field; Elasmo, elasmobranchs.
4 Discussion
The integrated approach applied in this study proved to be a tool capable of identifying the principal sectors and pressures that have the greatest cumulative effect on ecosystems of South Mid Atlantic Ridge, thereby measuring and ranking the possible prioritizing management actions. Our analysis indicated that the choice of descriptive statistical method (sum or average) used to rank the risk factors affected the ranking, thus our prioritization of impact risk, principally by Ecological Components elements. This is because the number of impact chains of each risk factor (e.g., oceanic pelagic, deep sea fish, slope rock & reef) may have had an influence on the ranking process. A rise in the number of impact chains may increase risk if the sum method is used but decrease risk if the average method is applied (Piet et al., 2017). Therefore, when only the average method is considered in the evaluation, the risk factor with more chains (links) reduces the IR score obtained (e.g., pelagic fish and demersal elasmobranchs, see Table 6), thus decreasing the probability that this factor will be considered by management actions.
The integrated approach applied in this study revealed, for both sum and average methods and proportional connectance, that fishing, shipping, wastewater service and tourism/recreation were the sectors with the highest connectance and impact risk in the South Mid-Atlantic Ridge islands of St Paul’s Rocks, Ascension and St Helena, mainly due to the fact that these activities take place throughout almost the whole area (Halpern et al., 2008; Halpern et al., 2012; Taconet et al., 2019), and have relationships with most of the ecological components. The area is an important shipping route, with intense traffic of recreational/tourism boats from ocean cruise ships to small boats navigating around the islands (Canelas et al., 2019; Rowlands et al., 2019; Drew et al., 2021).
As fishing displays an important risk to ecosystem components, it was expected that pressures directly linked to it (e.g., species extraction and bycatch) would also show relevant impacts in the study area. However, in our study only 30.2% of the impacts of this sector were directly related to bycatch and resource extraction, and 56% as a source of litter pressure (Table 7), such as lost or dumped gear and buoys from longline and trawler fisheries, and other waste from fishing crew. Even though it is a high-impact activity, most fishing in the region has been conducted on the high seas, catching large pelagic fish, such as swordfish and several tuna species (Arrizabalaga et al., 2019), thus limiting the links to pelagic ecosystem components only.
Although a large-scale Ascension Island MPA has been designated, there are only a few prohibited or restricted activities. Large-scale commercial fishing in any part of the MPA, as well as all types of fishing beyond 12 nautical miles of the island, is prohibited. However, the population of Ascension Island has a strong cultural and livelihood connection with the sea. Thus, there are some permitted activities in part of MPA, such as recreational fishing activities – from the coast or from small boats – and also sport fishing, due to the economy around tourism (Ascension Island Government, 2021). Recreational fishing is the unique economically relevant recreational activity in the island, and there are concerns about the contribution of recreational fisheries on fish population changes, as they often target vulnerable species of large bodied predators as trophy fishes (La Bianca et al., 2018). An integrated marine management assessment in Ascension Island showed that marine and recreational fishing were identified as one of the sectors with potential environmental impacts undermining ecosystem health (Hardman et al., 2022).
In the SPSPA region, fishing became particularly intense within the area of the multiple-use and the no-take MPA, with 152 active vessels identified (Magris, 2021). Ascension’s region of high effort (35,000–47,000 estimated hooks set per month), extends northwest of the EEZ to ~10°N, 35°W (Rowlands et al., 2019). Commercial fishing within the Ascension Island EEZ is prohibited since the publication of the Ascension Island marine management plan (Ascension Island Government, 2021).
Tourism and recreation in St Helena are also considered the key drivers for economic development, currently the main growth sector with high values associated with seasonal wildlife watching trips (e.g., scuba diving and whale shark watching) which demand to be managed carefully (Rees et al., 2016; Smith et al., 2019). Ascension Island has potential to offer high-value tourism based around scuba diving and wildlife watching charismatic animals such as turtles and whales. Contrastingly, tourism is not permitted in the St Peter and St Paul archipelago because of their status as a no take area in most of the emerged portion of the area. Cruise ships are occasionally observed navigating around the islands because it is a navigation route from eastern to western Africa.
The high impact of the shipping sector highlighted in the study area may be related to the known commercial shipping route passing through both Saint Helena and Ascension waters due to the supply of the islands (Saint Helena Government, 2016), as well as routine trading routes, crossing the EEZ of both Brazil and UK and ABNJ between the islands (from www.marinetraffic.com). Multiple pressures were assigned to marine transportation; in general, the environmental effects of shipping include air pollution, ballast water containing aquatic invasive species, spills (such as oil, dry bulk cargo, and hazardous substances), garbage management, sources of plastic debris, underwater noise, collision with marine fauna, ship groundings or sinkings, and widespread sediment contamination in ports and harbors (Jaügerbrand et al., 2019; Walker et al., 2019). Some regulations and treaties have been adopted and encouraged by different organizations to mitigate these impacts: for examples, the Convention on the Prevention of Marine Pollution by Dumping Wastes and Other Matters (London Protocol) — which was extended to Saint Helena and Ascension waters —, the International Maritime Organization (IMO), Convention for the Prevention of Pollution from Ships (MARPOL) and the United Nations Convention on the Law of the Sea (UNCLOS). However, it is recognized that shipping has unintended impacts on the environment (Walker et al., 2019). Therefore, the multiple pressures, in addition to several knowledge gaps regarding the ecological consequences of shipping impacts (Jaügerbrand et al., 2019) and the increasing number and size of ships operating (Erbe et al., 2020), make this sector one of the priorities for management and research.
Wastewater in the studied islands is correlated with human density as expected. Consequently, SPSPA with four people continuously inhabiting the local research station generate comparatively low water discharges, mostly from local, small sewage. St Helena with more than 4000 residents and Ascension with 800, have more and different sources of wastewater beyond sewage, with other sources from local urbanization structure (Ascension Island Government, 2021). Wastewater, depending on source and local structure for outflow, can aggregate different contaminants, becoming more harmful to biodiversity. We have considered possible impacts from contaminants to affect shallow habitats more intensely, however, depending on type of contaminants and oceanographic conditions, the deep seabed can also be contaminated (Jamieson et al., 2017). Currently, in Ascension Island there is a controlled discharge of waste and effluents, with permission being needed for new discharges inside the MPA (Ascension Island Government, 2021). Typical impacts from wastewater, however, include eutrophication, which can modify local primary producers by selecting a few dominant species causing bottom-up effects via the food chain (WormLotze, 2006). Many pathogens can be associated with wastewater via sewage and affect fish and invertebrates (Wear, 2019). The impacts associated with wastewater are considered chronic (i.e., continuous), which means changes in the community to a different steady state (Tuholske et al., 2021). However, although the spatial scale of effects is generally local, it will depend on size of outfalls and oceanographic conditions.
Litter was one of the main pressures in the study area, and was considered as main pressure in other sites, such as Ireland’s marine waters (Pedreschi et al., 2019). Litter was related mainly to fishing and shipping activities, as such sectors represent important sources of litter in the global seas (Walker et al., 1997; Morales-Caselles et al., 2021). Such activities are intense in our study sites, and litter has been described as affecting ecological processes (Barnes et al., 2018; Nunes et al., 2019). Despite initiatives to provide appropriate reception facilities for garbage from vessels in some ports, shipowners are still facing serious difficulties to find adequate facilities for reception of ship-generated litter (Carpenter and Mcgill, 2003; Walker, 2016). The intense fishing activity around Ascension, St Helena Island and Saint Peter and Saint Paul’s Archipelago most probably generate ghost fishing due to the abandoned, lost, or discarded fishing gear. Therefore, since plastic contains the longest durability among materials, its accumulation on nearby beaches and in the deep sea causes several consequences to the ecosystems combined with negative socio-economic effects (Thushari and Senevirathna, 2020). Given that the present study was evaluated in oceanic islands that host endemic species and provide important ecosystem services, it is urgent to characterize the dimension of this pressure to mitigate its negative impacts.
Our analysis indicated that sand extractions (Non-living Resources) were not being considered a high impact pressure. Although low significance, it is important to highlight this pressure, as the sector sand extraction was identified as a potential risk, during the process of the Marine Management Plan of St Helena Island due to being data-limited and with no regulation (Hardman et al., 2022). The lack of information regarding the impacts of sand extraction on the marine environment in St Helena was filled through an environmental risk assessment carried out by Mynott et al. (2021). This study concluded that with current extraction levels, the risk to the marine environment is low. However, if there is a variation in the amount of sand extraction or a change in the extraction site, it is necessary to carry out new studies to observe possible environmental impacts. On Ascension Island, mineral extraction, which includes mining activity and extractions of rock and minerals, is prohibited inside MPAs. Development activities require permission, including building structures and laying pipes (Ascension Island Government, 2021).
Both the military and electromagnetic field (EMF) sectors as possible disturbances still lack high confidence information. Data about the military sector is not easily accessible to scientists and to the public, as they involve confidential information. EMF are present everywhere in the oceans, and were considered by the stakeholders as an emergent topic, with possible impacts in the studied area. Undersea cables that are used for power transfer are well known sources of EMF, and they are rapidly increasing in number, capacity, and extent with the world exigences for electrical power generation (including wind energy), SMART grids, interconnector transmission and telecommunications (Hutchison et al., 2020a). Undersea communication cables are widely conspicuous on the seafloor and will be a near reality in the studied area. In this sense, two submarine cables were supposed to be constructed in the 2010’s close to Saint Helena (EQUIANO and SAEx); while the SAEx project was abandoned, EQUIANO is scheduled to be operational, probably by the end of 20222. They can generate electric currents and EMF, which could impact several marine species, mainly elasmobranchs, fishes, mammals, turtles, mollusks and crustaceans (Tricas and Gill, 2011). Impacts from submarine cables are considered as minor, if any, or of short term; however, uncertainty remains, especially related to the impacts of EMF after long exposures, as even when the cables are buried and well protected, the sediment layer and the cable wrapping structures do not prevent EMF from escaping (Taormina et al., 2018). Electrosensitive and magnetosensitive animals, like elasmobranchs, some bony fish and some invertebrates have the potential to suffer more with the influence of cable emissions. They could affect orientation, migration, communication, predatory behavior and other physiological processes (Tricas and Gill, 2011). EMF would cause a higher impact on organisms associated with the bottom, since EMF rapidly decrease with distance from the cable (Taormina et al., 2018). A recent study has shown that EMF from cables can change the exploratory behavior of the American lobster and the electro-sensitive little-skate in an enclosure environment (Hutchison et al., 2020a). Besides submarine cables being the main source of EMF for the marine environment, shipping and military ships can also generate EMF (Pawlowski, 2018), but the impact from these sources for the marine environment is still not evaluated. There is a need for studies on the ecological consequences of this emerging source of impact to prevent harm and to take measures to avoid them (Hutchison et al., 2020b).
When the impact of all sectors and pressures on ecological components was calculated we identified demersal and pelagic fish, and pelagic and demersal elasmobranchs as the most impacted. Considering demersal fish, the main impact risks are associated to dredging (e.g., abrasion, incidental loss, removal of substrates and sand), required to maintain access to Ascension’s and Santa Helena’s piers. Despite occurring rarely and with low spatial overlap, dredging affect the benthic production processes that are important to supporting demersal fish communities (Newell et al., 1998). Pressures linked to fishing, as abrasion (Kaiser et al., 2002), bycatch (Stobutzki et al., 2001), litter (Bellas et al., 2016; Alberghini et al., 2022) and species extraction (FAO, 2020; St Helena Government, 2022) also were recognized as drivers of changes in demersal species. However, if compared to coastal and continental areas, the risk of impact on demersal fish here was lower and with less intensity.
On the other hand, the effects of pressures on oceanic pelagic species in the study area is worrying. The main pressures linked with pelagic fish and elasmobranchs were litter, species extraction and contaminants. Threats on high-seas elasmobranchs population include overfishing, habitat degradation, pollution and entanglement (ghost fishing), with an elevated extinction risk (Dulvy et al., 2014; Queiroz et al., 2019). The whale shark (Rhincodon typus), for instance, has been threatened across its range leading to a conservation status of “Largely Depleted” on the IUCN Red List (Pierce et al., 2021). Even with the international protection and low fishing-induced mortality, Womersley et al. (2022) suggest that whale sharks deaths may be associated with hotspots of potential collision risk, due to overlapping cargo and tanker ships with seasonal sharks movements. The geographical range of whale sharks includes St. Peter and St. Paul's Rocks, Ascension, and St. Helena, and previous work reported putative reproductive behaviors and sexual activity in these oceanic islands (Hazin et al., 2008; Macena and Hazin, 2016; Perry et al., 2020). In addition, historical records indicated the ecological extinction of the Galapagos Shark population in Saint Paul’s Rocks (Luiz and Edwards, 2011). However, an increase in the number of these sharks was recently observed at St Paul's Rocks after the banishment of longline fishing in 2012 (Pimentel et al., 2022), as well as around Ascension (Burns et al., 2020). Thus, evidence suggests that the high-seas are particularly important and critical hotspot for sharks and rays, highlighting the importance of these regions where mitigation measures could be best focused.
A range of large pelagic fish are targeted by fishers and recreational fishing in the study area, such as Yellowfin tuna (Thunnus albacares), bigeye tuna (Thunnus obesus), Albacore (Thunnus alalunga), Blackfin tuna (Thunnus atlanticus), skipjack tuna (Katsuwonus pelamis), Frigate tuna (Auxis thazard), wahoo (Acanthocybium solandri), Swordfish (Xiphias gladius), Blue marlin (Makaira nigricans), Atlantic sailfish (Istiophorus albicans), Common dolphinfish (Coryphaena hippurus), among others (Ascension Island Government, 2021; Global Fishing Watch, 2021; ICCAT, 2022; St Helena Government, 2022). Conservation measures are in effect for certain large pelagic species, as bigeye tuna, swordfish and albacores, and the Regional Fisheries Management Organizations are responsible to manage some species (Joseph et al., 2019). However, there are no catch limit control rules for other small tuna species, such as common dolphinfish, skipjack tuna, wahoo and blackfin tuna, because there is little information available to determine the stock structure. Nevertheless, these species have a high socio-economic relevance for a considerable number of local communities at the regional level, which depend on landings for their livelihoods (ICCAT, 2020). Even with the management measures in force, the stocks of bigeye and yellowfin tuna are currently overfished and effective mitigation measures were necessary (Burns et al., 2020; ICCAT, 2022). However, fisheries pressures do not act in isolation and the potential cumulative effects of other sectors and their pressures, should also be considered. Some of our top ranked threats in relation to the pelagic fish group in the region were shipping (contaminants and litter) and tourism (sport fishing and litter).
Regarding deep sea habitat and species (> 750 m depth), the small risks of impact described by stakeholders may be related to the poor knowledge about this environment. There are exist large gaps in understanding not only human impacts to the Mid-South Atlantic, but also in basic knowledge of biology, ecology and fundamental life histories of species (Howell et al., 2020). Globally there is increasing evidence that mesophotic and deep-sea environments are already impacted by human activities from local to global levels from activities such as disposal of waste, litter, fisheries (Chiba et al., 2018; Rocha et al., 2018; Pinheiro et al., 2020), as well as climate change related impacts (Levin and Le Bris, 2015).
To conclude, it is clear that these seemingly pristine islands and oceanic waters already have been historically impacted by human activities (Luiz and Edwards, 2011; Burns et al., 2020), and this should be addressed by local conservation measures and international agreements. The potential environmental impacts of tourism and recreation, highlighted here, need to be monitored to preserve ecosystem health, mainly in Ascension and St Helena Islands due to the current growth of this sector. Local impacts of the wastewater sector and sand extraction industry deserve equal attention, mainly at St Helena, where one of the main anthropogenic risks to marine water quality is land-based run-off including untreated sewage (Saint Helena Government, 2016). For the whole area, this approach also highlights priorities for regulation and policy measures for fishing and shipping sectors, mainly for controlled litter disposal at sea, and identifies gaps in knowledge requiring research effort and policy interventions. Low risks of impact by electromagnetic fields to deep sea species/environments, and from the military sector, may be due to poor knowledge, indicating the need for future research, indicator development and monitoring.
The Integrated Ecosystem Assessment (IEA) presented in this study is essential to achieve a holistic perspective for identifying and understanding the complexity of interactions between different systems (such as coral reefs, islands and high seas) and multiple sectors, where users often may be in different jurisdictions regulated by different political systems, and with different values, arrangements and economic needs. The IEA process was carried out and validated together with the stakeholders of the study area, thus, through the results obtained, it is possible for stakeholders and managers to make use of the data to support the constant and adaptive process of managing MPAs. In addition, the ODEMM methodology, which was used to carry out the IEA, is an adaptable and repeatable methodology, and can be used in new situations, if necessary. As the risk assessment allows the identification of the most severe impact chain, it is possible to investigate how the management of some sectors or pressures can reduce the impact on the ecosystem. Thus, managers will have the necessary tools and resources to make fully informed decisions, being able to choose and evaluate management options based on the principles of Ecosystem-Based Management (Robinson et al., 2014).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
AR performed the analysis, produced the tables and figures and drafted the manuscript. DF helped with data analysis. AR, SF, VG, DF, VG, FS, AL, CF, MG contributed to assess all scores for each impact chain and to the in-house reviews. KH facilitated working with Ascension Island and St Helena Government and contributed to workshops. All authors discussed the results and contributed to the final manuscript. MG chaired the SOMAR stakeholders workshop and supervised AR. MG and SF coordinated the project. All authors contributed to the article and approved the submitted version.
Funding
AR thanks the University of Sao Paulo Foundation (FUSP) and the Oceanographic Institute (IOUSP) for the implementation of her postdoc fellowship. Long term ecological monitoring in the Brazilian oceanic islands is supported by CNPq 441327/2020-6 (PI: CF). This study is part of the EU HORIZON 2020-funded Mission Atlantic Project (Grant Agreement No 862428) for which we extend our acknowledgement to all partner institutions.
Acknowledgments
We thank Drs. Debbi Pedreschi and David Reid (MI, Ireland) for the methodological guidance, Drs. Olga Sato and Paulo Polito (USP, Brazil) for the constructive advice on ocean circulation, Dr. Tiago B.R Gandra (IFSC, Brazil) for sharing the R-codes and Drs. Amelia Bridges and Guilia la Bianca (UP, England) for the help in the literature review. We also acknowledge the stakeholders participants from several institutions that contributed during SOMAR workshop, and particularly the valuable input from Diane Baum (Ascension Island) and Rhys Hobb (St Helena Island) during the scoping validation phase.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1001676/full#supplementary-material
Footnotes
- ^ from www.marinetraffic.com
- ^ http://sainthelenaisland.info/communications.htm
References
Alberghini L., Truant A., Santonicola S., Colavita G., Giaccone V. (2022). Microplastics in fish and fishery products and risks for human health: A review. Int. J. Environ. Res. Public Health 20 (1), 789. doi: 10.3390/ijerph20010789
AORA (2019). Working group on the ecosystem approach to ocean health and stress-ors. linking ocean-use sectors and ecosystem components. (Copenhagen: Ices Expert Groups Reports), 56. doi: 10.17895/ices.pub.20291367
Arrizabalaga H., Santiago J., Granado I., Kroodsma D., Miller N. A., Fernandes J. A. (2019). “FAO area 47 – AIS-based fishing activity in the southeast Atlantic,” in Global atlas of AIS-based fishing activity – challenges and opportunities. Eds. Taconet M., Kroodsma D., Fernandes J. A. (Rome: FAO). Available at: www.fao.org/3/ca7012en/ca7012en.pdf.
Ascension Island Government (2021) The ascension island marine protected area management plan 2021–2026. Available at: https://www.ascension.gov.ac/project/mpa-management-plan.
Barnes D. K., Morley S. A., Bell J., Brewin P., Brigden K., Collins M., et al. (2018). Marine plastics threaten giant Atlantic marine protected areas. Curr. Biol. 28 (19), R1137–R1138. doi: 10.1016/j.cub.2018.08.064
Bellas J., Martínez-Armental J., Martínez-Cámara A., Besada V., Martínez-Gómez C. (2016). Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts. Mar. pollut. Bull. 109 (1), 55–60. doi: 10.1016/j.marpolbul.2016.06.026
Breen P., Robinson L. A., Rogers S. I., Knights A. M., Piet G., Churilova T., et al. (2012). An environmental assessment of risk in achieving good environmental status to support regional prioritisation of management in Europe. Mar. Policy 36, 1033–1043. doi: 10.1016/j.marpol.2012.02.003
Brown J., Beard A., Clingham E., Fricke R., Henry L., Wirtz P. (2019). The fishes of St Helena island, central Atlantic ocean – new records and an annotated check-list. Zootaxa 4543 (2), 151–194. doi: 10.11646/zootaxa.4543.2.1
Burns P., Hawkins J., Roberts C. (2020). Reconstructing the history of ocean wildlife around ascension island. Aquat. Conserv.: Mar. Freshw. Ecosyst. 30, 1220–1237. doi: 10.1002/aqc.3304
Canelas J., Fish R., Bormpoudakis D., Smith N. (2019). South Atlantic natural capital project: Cultural ecosystem services on ascension island. final report. natural capital assessment (South Atlantic Overseas Territories (Kent, UK: SAERI/University of Kent).
Carpenter A., Mcgill S. (2003). The EU directive on port reception facilities for ship generated waste and cargo residues: Current availability of facilities in the North Sea. Mar. Poll. Bull. 46, 21–32. doi: 10.1016/S0025-326X(02)00421-6
Chiba S., Saito H., Fletcher R., Yogi T., Kayo M., Miyagi S., et al. (2018). Human footprint in the abyss: 30 year records of deep-sea plastic debris. Mar. Pol. 96, 204–212. doi: 10.1016/j.marpol.2018.03.022
Cormier R., Kelble C. R., Anderson M. R., Allen J. I., Grehan A., Gregersen O. (2017). Moving from ecosystem-based policy objectives to operational implementation of ecosystem-based management measures. J. Mar. Sci. 74, 406–413. doi: 10.1093/icesjms/fsw181
Drew J., Andrews K., Smith N. (2021). The potential for expansion of whale shark (Rhincodon typus) tourism in St Helena: stakeholder engagement and willingness to pay. J. Ecotourism 21, 1–12. doi: 10.1080/14724049.2021.1971684
Duchez A., Courtois P., Harris E., Josey S. A., Kanzow T., Marsh R., et al. (2016). Potential for seasonal prediction of Atlantic sea surface temperatures using the RAPID array at 26°N. Clim. Dynam. 46, 3351–3370. doi: 10.1007/s00382-015-2918-1
Dulvy N. K., Fowler S. L., Musick J. A., Cavanagh R. D., Kyne P. M., Harrison L. R., et al. (2014). Extinction risk and conservation of the world's sharks and rays. eLife 3, e00590. doi: 10.7554/eLife.00590
Edwards A. J. (1990). Fish and fisheries of saint Helena island (Washington, Tyne & Wear: NB Print & Printsetting).
Edwards A. J., Lubbock H. R. (1983a). Marine zoogeography of saint paul's rocks. J. Biogeogr. 10, 65–72. doi: 10.2307/2844583
Edwards A. J., Lubbock H. R. (1983b). The ecology of saint paul's rocks (Equatorial Atlantic). J. Zool. 200, 51–69. doi: 10.1111/j.1469-7998.1983.tb06108.x
Erbe C., Smith J. N., Redfern J. V., Peel D. (2020). Editorial: Impacts of shipping on marine fauna. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00637
FAO (2020). Worldwide review of bottom fisheries in the high seas in 2016 (Rome: FAO Fisheries and Aquaculture Technical Paper No. 657). doi: 10.4060/ca7692en
Fletcher W. J. (2005). The application of qualitative risk assessment methodology to prioritize issues for fisheries management. ICES J. Mar. Res. 62, 1576–1587. doi: 10.1016/j.icesjms.2005.06.005
Fletcher P. J., Kelble C. R., Nuttle W. K., Kiker G. A. (2014). Using the integrated ecosystem assessment framework to build consensus and transfer information to managers. Ecol. Indic. 44, 11–25. doi: 10.1016/j.ecolind.2014.03.024
Fletcher W. J., Shaw J., Metcalf S. J., Gaughan D. J. (2010). An ecosystem based fisheries management framework: the efficient, regional-level planning tool for management agencies. Mar. Policy 34, 1226–1238. doi: 10.1016/j.marpol.2010.04.007
Floeter S. R., Rocha L. A., Robertson D. R., Joyeux J. C., Smith-Vaniz W. F., Wirtz P., et al. (2008). Atlantic Reef fish biogeography and evolution. J. Biogeogr. 35, 22–47. doi: 10.1111/j.1365-2699.2007.01790.x
Giglio V. J., Pinheiro H. T., Bender M. G., Bonaldo R. M., Costa-Lotufo L. V., Ferreira C. E. L., et al. (2018). Large And remote marine protected areas in the south Atlantic ocean are flawed and raise concerns: comments on soares and Lucas. Mar. Policy 96, 13–17. doi: 10.1016/j.marpol.2018.07.017
Global Fishing Watch (2021). Available at: https://globalfishingwatch.org/ (Accessed August 6, 2021).
Halpern B. S., Hengl T., Groll D. (2012) Shipping density (commercial). a global map of human impacts to marine ecosystems, showing relative density (in color) against a black background. Available at: https://commons.wikimedia.org/w/index.php?curid=18755723.
Halpern B. S., Walbridge S., Selkoe K., Kappel C., Micheli F., D'Agrosa, et al. (2008). A global map of human impact on marine ecosystems. Science 319 (5865), 948–952. doi: 10.1126/science.1149345
Hardman E., Thomas H. L., Baum D., Clingham E., Hobbs R., Stamford T., et al. (2022). Integrated marine management in the united kingdom overseas territories. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.643729
Hazin F., Vaske Júnior T., Oliveira P., Macena B., Carvalho F. (2008). Occurrences of whale shark (Rhincodon typus smit) in the saint Peter and saint Paul archipelago, brazil. braz. J. Biol. 68, 385–389. doi: 10.1590/s1519-69842008000200021
Hobday A. J., Smith A. D. M., Stobutzki I. C., Bulman C., Daley R., Dambacher J. M., et al. (2011). Ecological risk assessment for the effects of fishing. Fish. Res. 108, 372–384. doi: 10.1016/j.fishres.2011.01.013
Howell K. L., Hilário A., Allcock A. L., Bailey D. M., Baker M., Clark M. R., et al. (2020). A blueprint for an inclusive, global deep-sea ocean decade field program. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.584861
Hutchison Z. L., Gil A. B., Sigray P., He H., King J. W. (2020a). Anthropogenic electromagnetic fields (EMF) influence the behaviour of bottom-dwelling marine species. Sci. Rep. 10, 4219. doi: 10.1038/s41598-020-60793-x
Hutchison Z. L., Secor D. H., Gill A. B. (2020b). The interaction between resource species and electromagnetic fields associated with electricity production by offshore wind farms. Oceanogr 33 (4), 96–107. doi: 10.5670/oceanog.2020.409
ICCAT (International Commission for the Conservation of Atlantic Tunas) (2020). Report for biennial period 2018-19 part ii, (2019) - vol. 2 english version scrs Madrid, Spain: ICCAT Secretariat. Available at: https://www.iccat.int/documents/bienrep/rep_en_18-19_ii-2.pdf.
ICCAT (International Commission for the Conservation of Atlantic Tunas) (2022) Statistical bulletin. Vol.47. Madrid, Spain: CCAT Secretariat. Available at: https://www.iccat.int/sbull/SB47-2022/index.html (Accessed November, 2022).
ICES (2021). “AORA working group on the ecosystem approach to ocean health and stressors,” in Tools for ecosystem-based management (EA2OHS; outputs from 2018 meeting), vol. 3. (Copenhagen: ICES Scientific Reports), 81. doi: 10.17895/ices.pub.8230
IUCN (International Union for Conservation of Nature and Natural Resources) (2022) Governing areas beyond national jurisdiction. Gland, Switzerland: Issues Brief. Available at: https://www.iucn.org/sites/dev/files/issues_brief_governing_areas_beyond_national_jurisdiction.pdf.
Jaügerbrand A. K., Brutemark A., Svedeín J. B., Gren I. (2019). A review on the environmental impacts of shipping on aquatic and nearshore ecosystems. Sci. Total Environ. 695, 133637. doi: 10.1016/j.scitotenv.2019.133637
Jamieson A., Malkocs T., Piertney S., Fujii T., Zhang Z. (2017). Bioaccumulation of persistent organic pollutants in the deepest ocean fauna. Nat. Ecol. Evol. 1, 51. doi: 10.1038/s41559-016-0051
Joseph J., Shipley O. N., Siskey M. R. (2019). “Open ocean fisheries for Large pelagic species,” in Encyclopedia of ocean sciences, 3rd Edition. Eds. Cochran J. K., Bokuniewicz H. J., Yager P. L.. (Academic Press International).
Joyeux J., Floeter S. R., Ferreira C. E. L., Gasparini J. L. (2001). Biogeography of tropical reef fishes: the south Atlantic puzzle. J. Biogeogr. 28, 831–841. doi: 10.1046/j.1365-2699.2001.00602.x
Kaiser M. J., Collie J. S., Hall S. J., Jennings S., Poiner I. R. (2002). Modification of marine habitats by trawling activities: prognosis and solutions. Fish Fisheries 3, 114–136. doi: 10.1046/j.1467-2979.2002.00079.x
Knights A. M., Piet G. J., Jongbloed R. H., Tamis J. E., White L., Akoglu E., et al. (2015). An exposure-effect approach for evaluating ecosystem-wide risks from human activities. J. Mar. Sci. 72, 1105–1115. doi: 10.1093/icesjms/fsu245
La Bianca G., Tillin H., Hodgson B., Erni-cassola G., Howell K., Rees S. (2018). Ascension island- natural capital assessment: Marine ecosystem services report (Peterborough: JNCC).
Levin P. S., Fogarty M. J., Murawski S. A., Fluharty D. (2009). Integrated ecosystem assessments: Developing the scientific basis for ecosystem-based management of the ocean. PloS Biol. 7, e1000014. doi: 10.1371/journal.pbio.1000014
Levin P. S., Kelble C. R., Shuford R. L., Ainsworth C., DeReynier Y. (2014). Guidance for implementation of integrated ecosystem assessments: a US perspective. J. Mar. Sci. 71 (5), 1198–1204. doi: 10.1093/icesjms/fst112
Levin L. A., Le Bris N. (2015). The deep ocean under climate change. Science 350 (6262), 766–768. doi: 10.1126/science.aad0126
Luiz O. J., Edwards A. J. (2011). Extinction of a shark population in the archipelago of saint paul’s rocks (equatorial Atlantic) inferred from the historical record. Biol. Conserv. 144, 2873–2881. doi: 10.1016/j.biocon.2011.08.004
Luko C. D., Da Silveira I. C. A., Simoes-Sousa I. T., Araujo J. M., Tandon A. (2021). Revisiting the Atlantic south equatorial current. J. Geophys. Res. Oceans 126, e2021JC017387. doi: 10.1029/2021JC017387
Macena B. C., Hazin F. H. (2016). Whale shark (Rhincodon typus) seasonal occurrence, abundance and demographic structure in the mid-equatorial Atlantic ocean. PloS One 11, e0164440. doi: 10.1371/journal.pone.0164440
Magris R. A. (2021). Effectiveness of Large-scale marine protected areas in the Atlantic ocean for reducing fishing activities. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.711011
Marcello F., Wainer I., Rodrigues R. R. (2018). South Atlantic subtropical gyre late twentieth century changes. J. Geophys. Research: Oceans 123, 5194–5209. doi: 10.1029/2018JC013815
Möllmann C., Lindegren M., Blenckner T., Bergström L., Casini M., Diekmann R., et al. (2014). Implementing ecosystem-based fisheries management: from single-species to integrated ecosystem assessment and advice for Baltic Sea fish stocks. J. Mar. Sci. 71 (5), 1187–1197. doi: 10.1093/icesjms/fst123
Morales-Caselles C., Viejo J., Martí E., González-Fernández D., Pragnell-Raasch H., González-Gordillo J. I., et al. (2021). An inshore–offshore sorting system revealed from global classification of ocean litter. Nat. Sustainability 4 (6), 484–493. doi: 10.1038/s41893-021-00720-8
Muench A., Mengo E., Weber S., Baum D., Richardson A. J., Thomas H., et al. (2022). An assessment of economic viability of the ascension island tuna longline fishery management: Implications for marine protected area planning and future fisheries management. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.648437
Mynott F., Lonsdale J.-A., Stamford T. (2021). Developing an ecological risk assessment to effectively manage marine resources in data-limited locations: A case study for St Helena sand extraction. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.645225
Newell R. C., Seiderer L., Hitchcock D. R. (1998). The impact of dredging works in coastal waters: A review of the sensitivity to disturbances and subsequent recovery of biological resources in the seabed. Oceanogr. Mar. Biol. Annu. Rev. 36, 809–818.
Nunes L. T., Cord I., Francini-Filho R. B., Stampar S. N., Pinheiro H. T., Rocha L. A., et al. (2019). Ecology of prognathodes obliquus, a butterflyfish endemic to mesophotic ecosystems of St. Peter and St. Paul’s Archipelago. Coral Reefs 38 (5), 955–960.
O’Higgins T. G., DeWitt T. H., Lago M. (2020). “Using the concepts and tools of social ecological systems and ecosystem services to advance the practice of ecosystem-based management,” in Ecosystem-based management, ecosystem services and aquatic biodiversity, vol. 579 . Eds. O’Higgins T. G., Lago M., DeWitt T. H. (Springer, Cham: Springer Open). doi: 10.1007/978-3-030-45843-0_1
Pawlowski T. (2018). A review of electromagnetic field sources on ships. Bull. Maritime Inst. Gdansk 33 (1), 75–82. doi: 10.5604/01.3001.0012.7649
Pedreschi D., Bouch P., Moriarty M., Nixon E., Knights A., Reid D. (2019). Integrated ecosystem analysis in Irish waters; providing the context for ecosystem-based fisheries management. Fish. Res. 209, 218–229. doi: 10.1016/j.fishres.2018.09.023
Perry C. T., Clingham E., Webb D. H., de la Parra R., Pierce S. J., Beard A., et al. (2020). St. Helena: An important reproductive habitat for whale sharks (Rhincodon typus) in the central south Atlantic. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.576343
Pierce S. J., Grace M. K., Araujo G. (2021)Rhincodon typus (Green statuscassessment). In: The IUCN red list of threatened species 2021: e.T19488A1948820221 (Accessed 04 February 2023).
Piet G., Delacaímara G., Kraan M., Roückmann C., Lago M. (2020). “Advancing aquatic ecosystem-based management with full consideration of the social-ecological system,” in Ecosystem-based management, ecosystem services and aquatic biodiversity, vol. 579 . Eds. O’Higgins T. G., Lago M., DeWitt T. H. (Springer International Publishing). doi: 10.1007/978-3-030-45843-0_1
Piet G. J., Jongbloed R. H., Knights A. M., Tamis J. E., Paijmans A. J., van der Sluis M. T., et al. (2015). Evaluation of ecosystem-based marine management strategies based on risk assessment. Biol. Conser. 186, 158–166. doi: 10.1016/j.biocon.2015.03.011
Piet G. J., Knights A. M., Jongbloed R. H., Tamis J. E., de Vries P., Robinson L. A. (2017). Ecological risk assessments to guide decision-making: Methodology matters. Environ. Sci. Policy 68, 1–9. doi: 10.1016/j.envsci.2016.11.009
Pimentel C. R., Pinheiro H. T., Giarrizzo T., Francini-Filho R. B., Reis-Filho J. A., Rocha L. A., et al. (2022). Ecological Links between Pelagic and Mesophotic Reef Fishes in an Oceanic Archipelago of the Equatorial Atlantic Ocean. Diversity 14, 273. doi: 10.3390/d14040273
Pinheiro H. T., Macena B. C. L., Francini-Filho R. B., Ferreira C. E. L., Albuquerque F. V., Bezerra N. P. A., et al. (2020). Fish biodiversity of saint Peter and saint paul’s archipelago, mid-Atlantic ridge, Brazil: new records and a species database. J. Fish Biol. 97, 1143–1153. doi: 10.1111/jfb.14484
Popova E., Vousden D., Sauer W. H., Mohammed E. Y., Allain V., Downey-Breedt N., et al. (2019). Ecological connectivity between the areas beyond national jurisdiction and coastal waters: Safeguarding interests of coastal communities in developing countries. Mar. Policy 104, 90–102. doi: 10.1016/j.marpol.2019.02.050
Queiroz N., Humphries N. E., Couto A., Vedor M., Costa I., Sequeira A. M. M., et al. (2019). Global spatial risk assessment of sharks under the footprint of fisheries. Nature 572, 461–466. doi: 10.1038/s41586-019-1444-4
Rees S., Clingham E., Rodwell L., Glegg G., Collins M. (2016)Marine ecosystem services of St helena. part 2: Ecosystem service valuations, future development thresholds and management. In: A report for the environment and natural resources directorate (Marine Institute Plymouth University). Available at: https://core.ac.uk/download/pdf/153536074.pdf (Accessed May 27, 2021).
Robinson L. A., Culhane F. E. (2020). “Linkage frameworks: An exploration tool for complex systems in ecosystem-based management,” in Ecosystem-based management, ecosystem services and aquatic biodiversity. Eds. O’Higgins T., Lago M., DeWitt T. (Cham: Springer). doi: 10.1007/978-3-030-45843-0_11
Robinson L. A., Culhane F. E., Baulcomb C., Bloomfield H., Boehnke-Henrichs A., Breen P., et al. (2014). Towards delivering ecosystem-based marine management: The ODEMM approach. deliverable 17, EC FP7 project, (244273) ‘Options for delivering ecosystem-based marine management’ (Liverpool, UK: University of Liverpool), 96. Available at: https://odemm.com/sites/default/files/ODEMM%20Report_0.pdf.
Robinson L. A., White L., Culhane F., Knights A. M. (2013). ODEMM pressure assessment userguide V.2. ODEMM guidance document series No.4. EC FP7 project, (244273) ‘Options for delivering ecosystem-based marine management’ (Liverpool, UK: University of Liverpool) 12, 14. Available at: https://odemm.com/sites/default/files/Pressure%20Assessment%20Guide%20V2.pdf.
Rocha L. A., Pinheiro H. T., Shepherd B., Papastamatiou Y. P., Luiz O. J., Pyle R. L., et al. (2018). Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 361 (6399), 281–284. doi: 10.1126/science.aaq1614
Rogers A. D., Sumaila U. R., Hussain S. S., Baulcomb C. (2014). The high seas and us (Oxford, UK: Publishing institution: The Global Ocean Commission).
Rowlands G., Brown J., Soule B., Boluda P. T., Rogers A. D. (2019). Satellite surveillance of fishing vessel activity in the ascension island exclusive economic zone and marine protected area. Mar. Pol. 101, 39–50. doi: 10.1016/j.marpol.2018.11.006
Saint Helena Government (2016) St Helena Marine management plan. Available at: https://www.sainthelena.gov.sh/wp-content/uploads/2018/07/Marine-Management-Plan.pdf.
Samhouri J., Haupt A., Levin P., Link J., Shuford R. (2014). Lessons learned from developing integrated ecosystem assessments to inform marine ecosystem-based management in the USA. J. Mar. Sci. 71, 1205–1215. doi: 10.1093/icesjms/fst141
Samhouri J. F., Levin P. S. (2012). Linking land-and sea-based activities to risk in coastal ecosystems. Biol. Conserv. 145 (1), 118–129. doi: 10.1016/j.biocon.2011.10.021
Shackeroff J. M., Hazen E. L., Crowder L. B. (2009). “The oceans as peopled seascapes,” in Ecosystem-based management for the oceans. Eds. McLeod K., Leslie H. (Washington DC: Island Press), 392.
Smith N., Drew J., Andrews K., Stevens N. (2019). “St Helena Marine tourism values,” in Final report for the south Atlantic overseas territories natural capital assessment (Falkland Islands: South Atlantic Environmental Research Institute (SAERI).
St Helena Government (2022) St Helena Marine management plan. Available at: https://www.sainthelena.gov.sh/wp-content/uploads/2022/10/4070-St-Helena-MMP_FINAL.pdf (Accessed January, 2023).
Stobutzki I., Miller M., Brewer D. (2001). Sustainability of fishery bycatch: a process for assessing highly diverse and numerous bycatch. Environ. Conserv. 28 (2), 167–181. doi: 10.1017/S0376892901000170
Stramma L., England M. (1999). On the water masses and mean circulation of the south Atlantic ocean. J. Geophys. Res. 20, 863–883. 104. doi: 10.1029/1999JC900139
Taconet M., Kroodsma D., Fernandes J. A. (2019). Global atlas of AIS-based fishing activity - challenges and opportunities (Rome: FAO). Available at: www.fao.org/3/ca7012en/ca7012en.pdf.
Talley L. D., Pickard G. L., Emery W. J., Swift J. H. (2011). “Chapter 9 - Atlantic ocean,” in Descriptive physical oceanography (6th edition). Eds. Talley L. D., Pickard G. L., Emery W. J., Swift J. H. (Boston: Academic Press), 245–301.
Taormina B., Bald J., Want A., Thouzeau G., Lejart M., Desroy N. and Carlier A. (2018). A review of potential impacts of submarine power cables on the marine environment: Knowledge gaps, recommendations and future directions. Renew.Sust. Energ. Rev. 96, 380–391. doi: 10.1016/j.rser.2018.07.026
Thushari G. G. N., Senevirathna J. D. M. (2020). Plastic pollution in the marine environment. Heliyon 6 (8), e04709. doi: 10.1016/j.heliyon.2020.e04709
Tricas T., Gill A. (2011). Effects of EMFs from undersea power cables on elasmobranchs and other marine species (Camarillo, CA: U.S. Dept. of the Interior, Bureau of Ocean Energy Management, Regulation, and Enforcement, Pacific OCS Region). OCS Study BOEMRE 2011-09.
Tuholske C., Halpern B. S., Blasco G., Villasenor J. C., Frazier M., Caylor K. (2021). Mapping global inputs and impacts from of human sewage in coastal ecosystems. PloS One 16 (11), e0258898. doi: 10.1371/journal.pone.0258898
Walker T. R. (2016). Green marine: An environmental program to establish sustainability in marine transportation. Mar. pollut. Bull. 105 (1), 199–207. doi: 10.1016/j.marpolbul.2016.02.029
Walker T. R., Adebambo O., Feijoo M. C. D. A., Elhaimer E., Hossain T., Edwards S. J., et al. (2019). “Environmental effects of marine transportation,” in World seas: an environmental evaluation (Academic Press International). doi: 10.1016/B978-0-12-805052-1.00030-9
Walker T. R., Reid K., Arnould J. P. Y., Croxall J. P. (1997). Marine debris surveys at bird island, south Georgia 1990–1995. Mar. pollut. Bull. 34 (1), 61–65. doi: 10.1016/S0025-326X(96)00053-7
Wear S. L. (2019). Battling a common enemy: Joining forces in the fight against sewage pollution. Bioscience 69 (5), 360–367. doi: 10.1093/biosci/biz025
Wirtz P., Bingeman J., Bingeman J., Fricke R., Hook T. J., Young J. (2014). The fishes of ascension island, central Atlantic ocean – new records and an annotated checklist. J. Mar. Biol. Assoc. 97 (4), 783–798. doi: 10.1017/S0025315414001301
Womersley F. C., Humphries N. E., Queiroz N., Vedor M., da Costa I., Furtado M., et al. (2022). Global collision-risk hotspots of marine traffic and the world’s largest fish, the whale shark. Proc. Natl. Acad. Sci. 119 (20), e2117440119. doi: 10.1073/pnas.2117440119
Keywords: integrated assessment, ecosystem indicators, multiple stressors, tropical islands, high seas, anthropogenic activities, impact risk
Citation: Rodrigues AR, Floeter SR, Gomes V, Ferrari DS, Giglio VJ, Silva FC, Liedke AMR, Ferreira CEL, Howell K and Gasalla MA (2023) Integrated ecosystem assessment around islands of the tropical South Mid-Atlantic Ridge. Front. Mar. Sci. 10:1001676. doi: 10.3389/fmars.2023.1001676
Received: 23 July 2022; Accepted: 10 March 2023;
Published: 04 April 2023.
Edited by:
Daniele Iudicone, Anton Dohrn Zoological Station Naples, ItalyReviewed by:
Suprapto Suprapto, Yogyakarta State University, IndonesiaBrandon Muffley, Mid Atlantic Fishery Management Council, United States
Copyright © 2023 Rodrigues, Floeter, Gomes, Ferrari, Giglio, Silva, Liedke, Ferreira, Howell and Gasalla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda R. Rodrigues, aricci@alumni.usp.br
 Amanda R. Rodrigues
Amanda R. Rodrigues Sergio R. Floeter2
Sergio R. Floeter2  Vicente Gomes
Vicente Gomes Débora S. Ferrari
Débora S. Ferrari Vinicius J. Giglio
Vinicius J. Giglio Fernanda C. Silva
Fernanda C. Silva Ana M. R. Liedke
Ana M. R. Liedke Carlos E. L. Ferreira
Carlos E. L. Ferreira Kerry Howell
Kerry Howell Maria A. Gasalla
Maria A. Gasalla