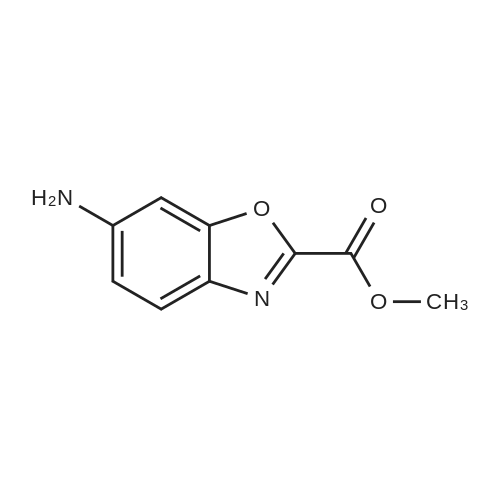
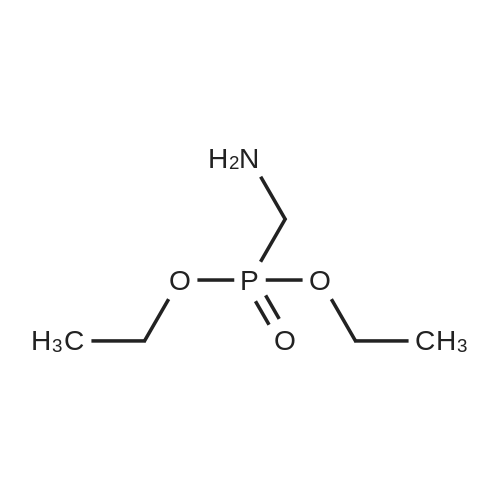
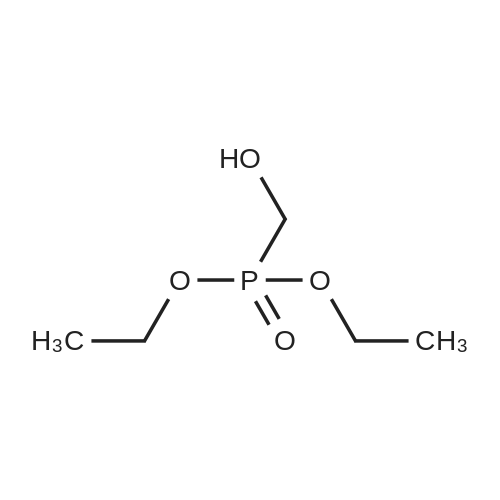
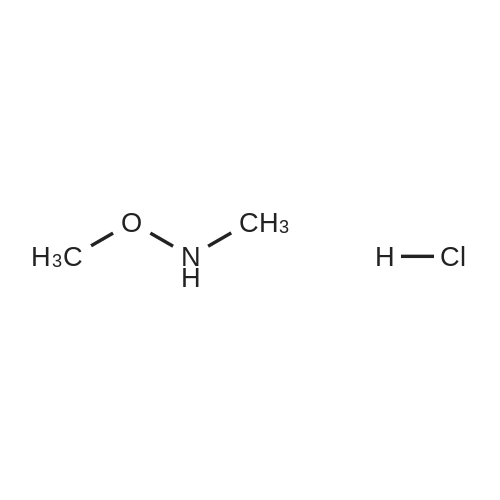
| 73% |
|
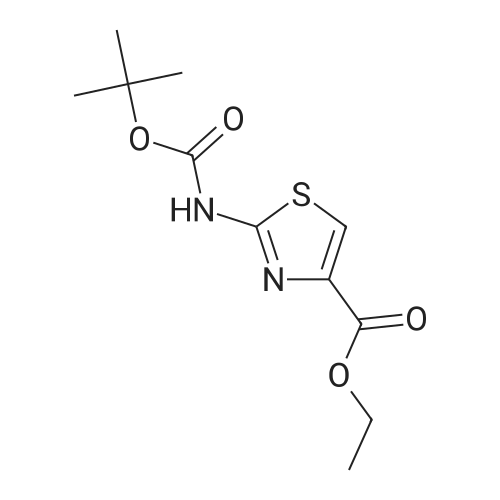
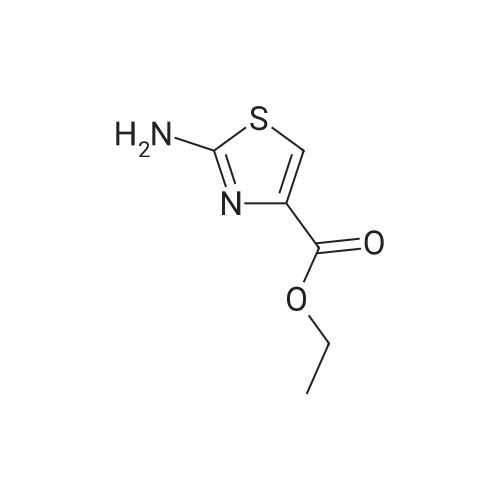
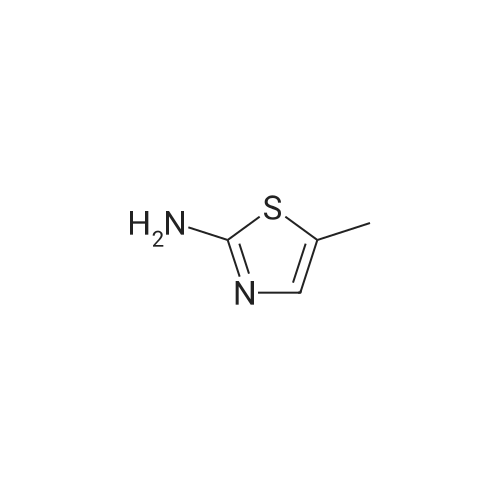
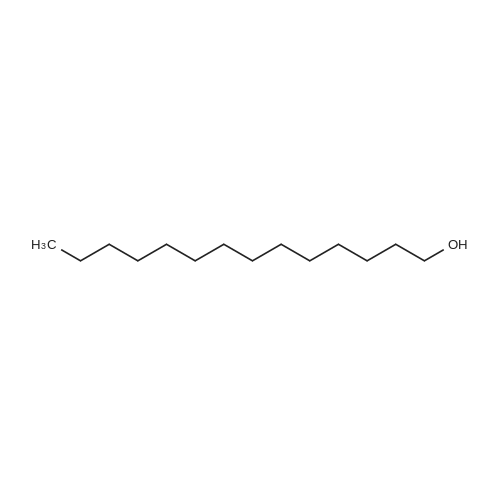
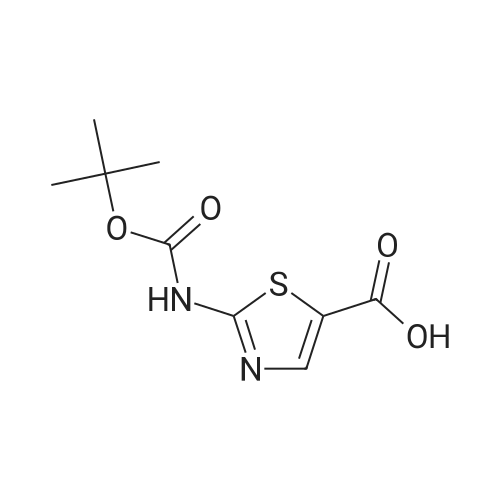
Thiourea (0.76 g, 10.0 mmol) was added to ethanol (10 mL), stirred at room temperature for 10 min, and ethyl bromopyruvate (1.25 mL, 10.0 mmol) was slowly added, at which time heat release was intense. The reaction mixture was stirred at 78C for 4 h, and the reaction completion was monitored by TLC. The reaction mixture was filtered over Celite and the ethanol layer was concentrated under reduced pressure to give pale-yellow solids. The crude product was recrystallized by ethyl acetate/cyclohexane to obtain intermediate (2) as yellow crystal (2.3g, 91%). 1H NMR (400 MHz, DMSO): delta 7.45 (s, 1H), 7.20 (s, 2H), 4.18 (q, J = 7.2 Hz, 2H), 1.24 (t, J = 7.2 Hz, 3H). ESI-MS (m/z): [M+H]+= 173.0. The intermediate (2) (1.42 g, 5.1 mmol) was dissolved in anhydrous pyridine (30 mL), and (Boc)2O (1.84 g, 8.4 mmol) was added, the mixture was refluxed for 6 h. Pyridine was removed by vacuum evaporation, the residua was diluted with ethyl acetate (80 mL), followed by washed with saturated NaCl solution three times. The organic phase was dried over Na2SO4, filtered, and then concentrated. The crude mixture was purified by column chromatography on silica gel to afford intermediate (3) as a white solid (0.83 g, 54%). 1H NMR (400 MHz, CDCl3): delta 8.32 (s, 1H), 7.79 (s, 1H), 4.38 (q, J = 7.1 Hz, 2H), 1.53 (s, 9H), 1.38 (t, J = 7.1 Hz, 3H). The intermediate (3) (200 mg, 0.7 mmol) was dissolved in ethanol (2 mL), and 1 M lithium hydroxide solution (2.1 mmol, 2.1 mL) was added dropwise at 0. The reaction mixture was stirred at room temperature for 4 h and then removed methanol under reduced pressure. The residue was acidified with 1 M HCl (pH~2) and then extracted with ethyl acetate three times. The combined organic phase was washed with brine and dried with Na2SO4. The organic phase is evaporated by rotary evaporation to afford the intermediate (4) as white solid and was used in the next step without futher purification and characterization. The intermediate (4) (74 mg, 0.3 mmol) was dissolved in tetrahydrofuran (6 mL), and DIPEA (100 uL, 0.6 mmol), 2,4, 6-trichlorobenzoyl chloride (71 uL, 0.5 mmol) were successively added in at 0, and the mixture was stirred at 0 for 30 min. 1-tetradecanol (65 mg, 0.3 mmol) and DMAP (74 mg, 0.6 mmol) were added, and the reaction mixture was stirred 0 for 1 hour and at room temperature for 1 hour. 1 M HCl solution (2 mL ) and water (12 mL) was added to the reaction mixture, the aqueous phase was extracted three times with ethyl acetate. The organic phase was dried over Na2SO4 , filtered, and then concentrated. The crude mixture was purified by column chromatography on silica gel to afford intermediate (5) as white solid (100 mg, 73%). 1H NMR (400 MHz, CDCl3): delta 8.53 (s, 1H), 7.75 (s, 1H), 4.30 (t, J = 6.8 Hz, 2H), 1.77-1.69 (m, 2H), 1.43 -1.34 (m, 2H), 1.31-1.18 (m, 20H), 0.87 (t, J = 6.8 Hz, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping