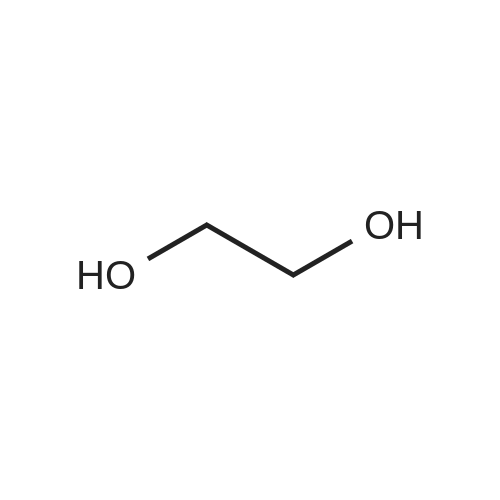
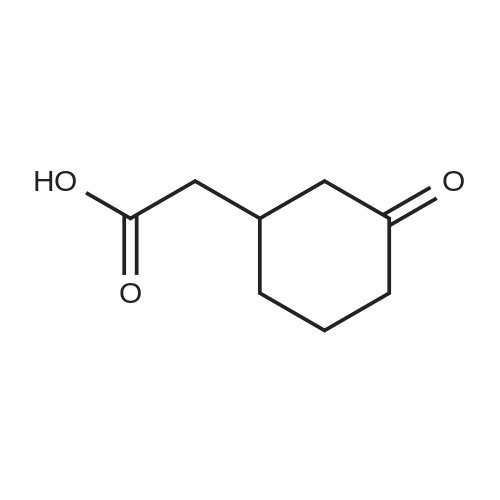
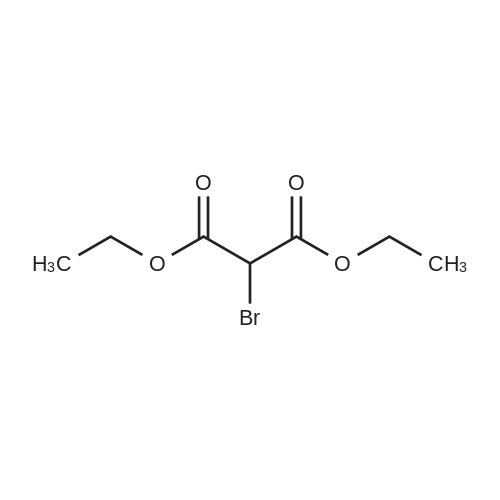
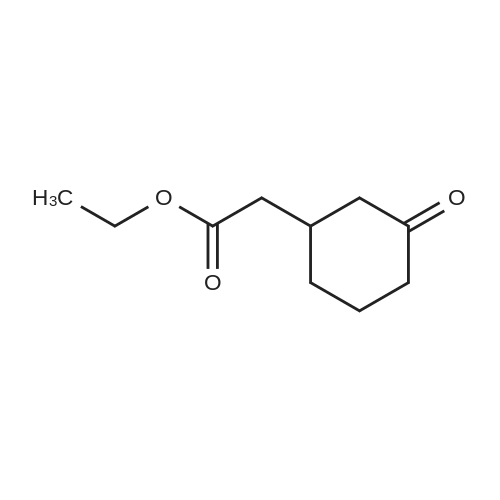
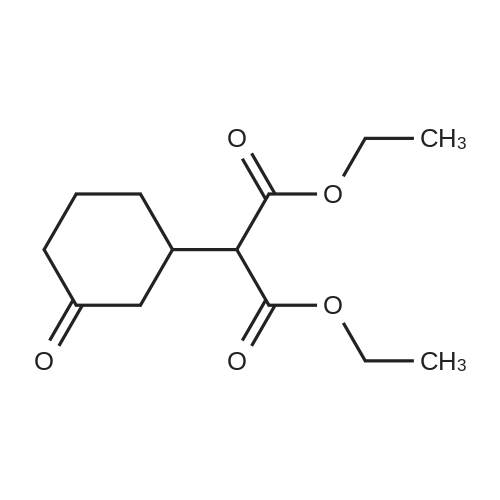
| 92% |
Stage #1: cyclohexenone; diethyl malonate With sodium hydride In diethyl ether at 20℃;
Stage #2: With hydrogenchloride In diethyl ether; water |
|
| 90% |
With 1-butyl-3-methylimidazolium hydroxide at 20℃; for 4h; |
|
| 90% |
With 1-butyl-3-methylimidazolium hydroxide at 20℃; for 4h; |
|
| 90% |
In acetonitrile at 25℃; for 1.5h; |
3.d
The catalyst obtained in example 1 was utilised to catalyse Michael's reactions by global budget/balance: Table 2, hereunder, groups the results observed for different kinds of acceptors and donors. The corresponding reactions (a) to (k) were achieved through reaction at a temperature of 25° C., under agitation and in 5 ml of dry acetonitril, 1 millimole of an acceptor like compound and 1 millimole of a donor like compound, in the presence of 0.1 g of ‘HDT-F’ catalyst prepared in example 1. |
| 90% |
With lithium perchlorate; triethylamine at 20℃; for 1h; Inert atmosphere; |
|
| 89% |
With nickel(II) ferrite In ethanol; water Heating; Green chemistry; |
|
| 87% |
With lithium aluminium tetrahydride; bis N-<(1R)-1-hydroxyphenylethan-2-yl> benzylamine In tetrahydrofuran at 20℃; for 6h; |
|
| 82% |
With potassium carbonate In dichloromethane at 20℃; |
|
| 63% |
With benzoic acid In water at 20℃; for 48h; |
E1. General Procedure for β-Functionalization of Enones
General procedure: A 4 mL glass vial was charged with the appropriate nucleophile 3a-e or 2a (0.1 mmol, 1 equiv.), NCDs-1 (3.6% w/v, 14.4mg), the appropriate enone 1a-d (0,3 mmol, 3 equiv.), the corresponding acid (HA, 20 mol%) and solvent (finalconcentration: 0.25 M). The resulting mixture was stirred for the indicated time (generally 48 hours) at ambient temperature.The reaction crude was then extracted with ethyl acetate and the organic phase was filtered through sodium sulfate. Thesolvent was removed under reduced pressure and the residue was purified by column chromatography (eluent: hexane/ethylacetate) to give the corresponding β-functionalized carbonyl compounds 4a-h. TFA (HA) and milli-Q water/dioxane (1:1)were used for the synthesis of compounds 4a-b. Benzoic acid (HA) and pure milli-Q water were used for the synthesis ofcompounds 4c-h. |
| 49% |
With tetrabutylammonium borohydride In methanol |
|
| 32% |
With lipozyme TLIM In water; dimethyl sulfoxide at 35℃; for 72h; Enzymatic reaction; |
|
| 30% |
With sodium carbonate for 5h; |
|
| 15% |
In acetonitrile at 79.9℃; for 60h; |
|
|
With sodium In diethyl ether; ethanol |
|
| 89 % Chromat. |
In 1,4-dioxane at 60℃; for 2h; |
|
| 89 % Chromat. |
In 1,4-dioxane at 60℃; for 2h; other catalysts, other solvents; |
|
|
With triethylamine; magnesium chloride 1.) acetonitrile, 2 h, 2.) acetonitrile, RT; Yield given. Multistep reaction; |
|
|
With N,N,N,N,N,N-hexamethylphosphoric triamide; lithium chloride 1.) RT, 15 min, 2.) 90 deg C, 3 h; Yield given. Multistep reaction; |
|
|
With sodium In ethanol |
1 Example 1
A solution of 30 ml of 2-cyclohexen-1-one in 35 ml anhydrous ethanol is added dropwise at a temperature below -10° to a reagent prepared from 0.1 g sodium, 100 ml of absolute ethanol and 50 ml of diethyl malonate. The reaction mixture is allowed to warm slowly and is stirred at room temperature overnight. The mixture is evaporated to dryness, the residue is dissolved in ether, the ether solution is washed with brine, dried, filtered and evaporated to dryness. The residue is distilled under reduced pressure to yield diethyl 3-oxocyclohexylmalonate, b.p. 147°/0.5 mm. |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene |
|
| 8 g |
With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 50℃; for 16h; |
Intermediate 45A: Diethyl 2-(3-oxocyclohexyl)malonate
Intermediate 45A: Diethyl 2-(3-oxocyclohexyl)malonate A solution of cyclohex-2-enone (3.05 mL, 30 mmol) and diethyl malonate (4.58 mL, 30.0 mmol) in THF (30 mL) was treated with 1,8-diazabicyclo[5.4.0]undec-7-ene (4.52 mL, 30.0 mmol) and heated at 50° C. for 16 h. The cooled mixture was poured into EtOAc and washed sequentially with 1M aqueous HCl and brine. The combined aqueous layers were extracted with EtOAc, and the combined organic phases were dried and concentrated to give diethyl 2-(3-oxocyclohexyl)malonate as an oil (8.0 g), used without further purification. Mass spectrum m/z 257 (M+H)+. |
|
With sodium ethanolate In tetrahydrofuran at 20℃; |
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping