Alternatived Products of [ 21753-16-2 ]
Product Details of [ 21753-16-2 ]
| CAS No. : | 21753-16-2 |
MDL No. : | MFCD01850992 |
| Formula : |
C12H11N3O2
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | - |
| M.W : |
229.24
|
Pubchem ID : | - |
| Synonyms : |
|
Safety of [ 21753-16-2 ]
| Signal Word: | |
Class: | |
| Precautionary Statements: | |
UN#: | |
| Hazard Statements: | |
Packing Group: | |
Application In Synthesis of [ 21753-16-2 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 21753-16-2 ]
- 1
-
 [ 117490-34-3 ]
[ 117490-34-3 ]

-
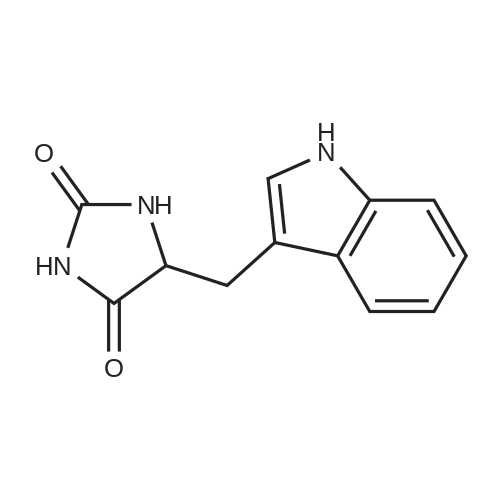 [ 21753-16-2 ]
[ 21753-16-2 ]
- 2
-
 [ 21753-16-2 ]
[ 21753-16-2 ]

-
 [ 105-36-2 ]
[ 105-36-2 ]

-
 [ 109498-12-6 ]
[ 109498-12-6 ]
- 3
-
 [ 21753-16-2 ]
[ 21753-16-2 ]

-
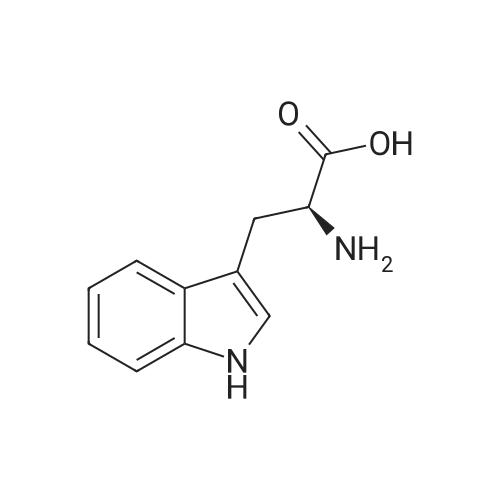 [ 73-22-3 ]
[ 73-22-3 ]
- 6
-
5-<indolyl-(3)-methylene>-hydantoin
[ No CAS ]

-
 [ 21753-16-2 ]
[ 21753-16-2 ]
- 7
-
 [ 21753-16-2 ]
[ 21753-16-2 ]

-
aq. barium hydroxide solution
[ No CAS ]

-
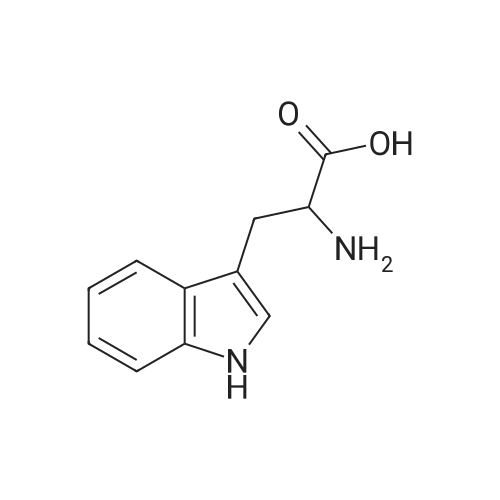 [ 54-12-6 ]
[ 54-12-6 ]
- 8
-
 [ 1118-02-1 ]
[ 1118-02-1 ]

-
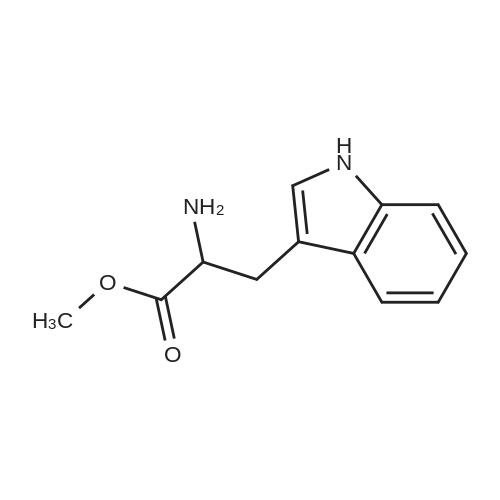 [ 7303-49-3 ]
[ 7303-49-3 ]

-
 [ 21753-16-2 ]
[ 21753-16-2 ]
- 9
-
5-(1H-indol-3-ylmethyl)-imidazolidine-2,4-dione potassium salt
[ No CAS ]

-
 [ 2290-65-5 ]
[ 2290-65-5 ]

-
 [ 21753-16-2 ]
[ 21753-16-2 ]
| Yield | Reaction Conditions | Operation in experiment |
| 93% |
With triethylamine; In dichloromethane; for 5.0h; |
To a solution of L-tryptophan methyl ester hydrochloride (0.500 mg, 0.002 mol.) in dichloromethane (10 mL) was added triethyl amine (3.0 mL) followed by trimethylsilylisocyanate (2.300 gm, 0.02 mol.). The reaction mixture was stirred at room temperature for 24 hr and then concentrated. The residue obtained was dissolved in AcOH (5 mL) and refluxed for 5 hr. The reaction mixture was extracted in ethyl acetate, washed with water, dried and concentrated. The residue was dissolved in EtOH and treated with KOH, and stirred for 30 min. Then the reaction was concentrated and dried under vacuum to give 5-(1H-indol-3-ylmethyl)-imidazolidine-2,4-dione potassium salt (245 mg, 46%). This solid (50 mg, 0.18 mg) was acidified with dilute HCl at 0 C. The residue was extracted in ethyl acetate, and concentrated. The solid obtained after concentration was dried under vacuum to give 893-19 (42 mg, 93%) |
- 10
-
C2 H5 OH
[ No CAS ]

-
NaOC2 H5
[ No CAS ]

-
 [ 21753-16-2 ]
[ 21753-16-2 ]

-
 [ 7732-18-5 ]
[ 7732-18-5 ]

-
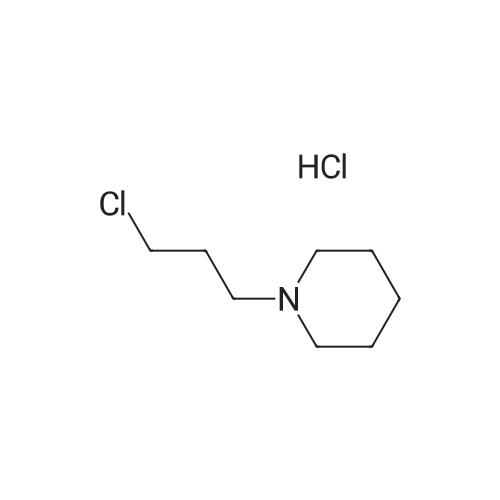 [ 5472-49-1 ]
[ 5472-49-1 ]

-
5-(Indol-3-ylmethyl)-3-(3-piperidylpropyl)hydantoin
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
In N,N-dimethyl-formamide; |
EXAMPLE 20 5-(Indol-3-ylmethyl)-3-(3-piperidylpropyl)hydantoin A mixture of 5-(indol-3-ylmethyl)hydantoin (10 g, 0.043 mole), N-(3-chloropropyl)piperidine hydrochloride (8.6 g, 0.043 mole), NaOC2 H5 (0.086 mole), 350 ml of anhydrous C2 H5 OH and 100 ml DMF was heated to reflux with stirring for 6 hours and poured into 1.5 l of H2 O. The solid was collected and recrystallized from aqueous methanol, yield 11.7 g, mp 185. ANALYSIS-- Calculated for C20 H26 N4 O2: C, 67.78; H, 7.39; N, 15.81. Found: C, 67.45; H, 7.32; N, 15.64. |
- 11
-
 [ 21753-16-2 ]
[ 21753-16-2 ]

-
 [ 75-64-9 ]
[ 75-64-9 ]

-
 [ 106-89-8 ]
[ 106-89-8 ]

-
 [ 69244-12-8 ]
[ 69244-12-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In C2 H5 OH; NaOC2 H5; |
EXAMPLE 19 3-(3-t-Butylamino-2-hydroxypropyl)-5-(indol-3-ylmethyl)hydantoin A mixture of 5-(indol-3-ylmethyl)hydantoin (11.5 g, 0.05 mole) in NaOC2 H5 (0.05 mole) and 300 ml of anhydrous C2 H5 OH was heated to reflux and 20 g of epichlorohydrin were added. The mixture was heated to reflux for 7 hours, filtered and concentrated in vacuo to an oil. The concentrate and t-butylamine (10 ml) in 100 ml of anhydrous C2 H5 OH were heated to reflux for 3 hours and concentrated in vacuo. The free base crystallized in ether and was recrystallized from 2-propanol-petroleum ether. |
- 12
-
NaOC2 H5
[ No CAS ]

-
 [ 15037-34-0 ]
[ 15037-34-0 ]

-
 [ 21753-16-2 ]
[ 21753-16-2 ]

-
 [ 69243-99-8 ]
[ 69243-99-8 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In C2 H5 OH; N,N-dimethyl-formamide; |
EXAMPLE 9 5-(Indol-3-ylmethyl)-3-[3-(4-phenyl-1-piperidyl)propyl]hydantoin A mixture of 5-(indol-3-ylmethyl)hydantoin (6.4 g, 0.028 mole), 1-(3-chloropropyl)-4-phenylpiperidine (6.6 g, 0.028 mole) and 200 ml of DMF in 200 ml of anhydrous C2 H5 OH and 0.028 mole of NaOC2 H5 was heated under reflux with stirring for 10 hours. The mixture was filtered and the filtrate was diluted with H2 O. The solid was collected and twice recrystallized from aqueous C2 H5 OH, yield 2.0 g, mp 179-80. ANALYSIS-- Calculated for C26 H30 N4 O2: C, 72.53; H, 7.02; N, 13.01. Found: C, 72.02; H, 7.06; N, 12.75. |
- 13
-
C2 H5 OH
[ No CAS ]

-
NaOC2 H5
[ No CAS ]

-
 [ 21753-16-2 ]
[ 21753-16-2 ]

-
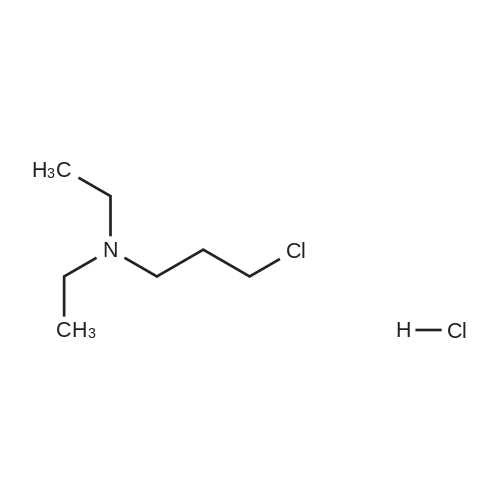 [ 4535-85-7 ]
[ 4535-85-7 ]

-
 [ 69244-09-3 ]
[ 69244-09-3 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In chloroform; |
EXAMPLE 16 3-(3-Diethylaminopropyl)-5-(indol-3-ylmethyl)hydantoin A mixture of 5-(indol-3-ylmethyl)hydantoin (10 g, 0.04 mole), 3-chloro-N,N-diethylpropylamine hydrochloride (8.2 g, 0.04 mole), NaOC2 H5 (0.08 mole) and 500 ml of anhydrous C2 H5 OH was heated to reflux with stirring for 20 hours. The mixture was filtered and concentrated to dryness. The concentrate was dissolved in CHCl3, filtered and concentrated. The concentrate was crystallized and recrystallized from benzene-petroleum ether. The solid was again recrystallized from xylene and the solvated solid was dried in vacuum, yield 2.5 g, mp 90. ANALYSIS-- Calculated for C19 H26 N4 O2: C, 66.64; H, 7.65; N, 16.36. Found: C, 66.04; H, 7.62; N, 15.99. |
- 14
-
NaOC2 H5
[ No CAS ]

-
1-(3-chloropropyl)-morpholine
[ No CAS ]

-
 [ 21753-16-2 ]
[ 21753-16-2 ]

-
 [ 69243-93-2 ]
[ 69243-93-2 ]
| Yield | Reaction Conditions | Operation in experiment |
|
In C2 H5 OH; |
EXAMPLE 5 5-[(Indol-3-yl)methyl]-3-(3-morpholinopropyl)hydantoin 5-[(Indol-3-yl)methyl]hydantoin (5 g; 0.022 mole) was added to NaOC2 H5 (0.022 mole) in 300 ml of anhydrous C2 H5 OH. The first mixture was heated to reflux, and 1-(3-chloropropyl)-morpholine (3.5 g, 0.022 mole) was added. The mixture was heated for 6 hours and then diluted with H2 O. The solid was collected and recrystallized from 2-propanol-petroleum ether, yield 3 g, mp 158-60. ANALYSIS-- Calculated for C19 H25 ClN4 O3: C, 59.09; H, 6.41; N, 14.26. Found: C, 57.50; H, 6.46; N, 13.96. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping










