Abstract
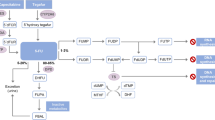
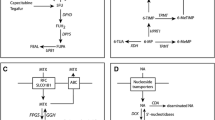
Fluorouracil is used clinically against various solid tumours. Both fluorouracil toxicity and pharmacokinetics vary highly within and between individuals. The reasons why doses are not individualised routinely are difficulties in defining, predicting and achieving an optimal fluorouracil exposure or dose because of a narrow therapeutic index, nonlinear pharmacokinetics, variabilities in administration rates and metabolism, and in targets like thymidylate synthase. To individualise fluorouracil administration before the first dose, assessment of the individual dihydropyrimidine dehydrogenase (DPD) activity may be useful, because this genetically highly polymorphic enzyme controls approximately 80% of fluorouracil elimination. A complete or partial loss of DPD activity in 0.1 and 3-5% of Caucasians, respectively, leads to increased fluorouracil exposure and toxicity. Several methods to assess DPD activity in patients have been proposed (genotyping, various phenotyping methods), but each of them has limitations, as has the fluorouracil test dose approach. To adapt exposure towards fluorouracil a priori, a combination of genotyping and phenotyping may yield better prediction of toxicity than one method alone.
A prerequisite for dose adaptation is the definition of fluorouracil exposure ranges with sufficient therapeutic activity, but without serious toxicity. While an increased risk of leukopenia, diarrhoea, stomatitis, and hand-foot syndrome during continuous 5-day infusions was related to fluorouracil exposures above an area under the plasma concentration-time curve (AUC) threshold of 25–30 mg · h/L, tumour response was higher when an AUC of approximately 30 mg · h/L was achieved, illustrating the extremely narrow therapeutic window of fluorouracil. Pharmacokinetic target values are less clear for other regimens, including chronomodulated regimens, which yielded a superior clinically efficacy and tolerability in several trials. However, the monitoring of fluorouracil plasma concentrations seems principally useful for individual a posteriori dose adjustment. Whether an adaptation of the fluorouracil starting dose to the results of two DPD activity tests before fluorouracil administration a priori, and the adaptation of doses to fluorouracil exposure a posteriori is a reasonable approach to better prevent toxicity and increase efficacy, remains to be evaluated in randomised clinical studies comparing these strategies to routine clinical safety monitoring.







Similar content being viewed by others
References
Linder MW, Prough RA, Valdes RJ. Pharmacogenetics: a laboratory tool for optimizing therapeutic efficacy. Clin Chem 1997; 43: 254–66
Shi MM, Bleavins VR, de la Iglesia FA. Technologies for detecting genetic polymorphism in pharmacogenomics. Mol Diagn 1999; 4: 343–51
Zühlsdorf MT. Relevance of pheno- and genotyping in clinical drug development. Int J Clin Pharmacol Ther 1998; 36: 607–12
Gill S, Thomas RR, Goldberg RM. Review article: colorectal cancer chemotherapy. Aliment Pharmacol Ther 2003; 18: 683–92
Wilke HJ, van Cutsem E. Current treatments and future perspectives in colorectal and gastric cancer. Ann Oncol 2003; 14 Suppl. 2: ii49–55
Braun AH, Achterrath W, Wilke H, et al. New systemic frontline treatment for metastatic colorectal carcinoma. Cancer 2004; 100: 1558–77
Ilson DH. Oesophageal cancer: new developments in systemic therapy. Cancer Treat Rev 2003; 21: 525–32
Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol 2004; 22: 2214–32
Anonymous. Drugs of choice for cancer chemotherapy. Med Lett Drugs Ther 1997; 39: 21–8
Milano G, Roman P, Khater P, et al. Dose versus pharmacokinetics for predicting tolerance to 5-day continuous infusion of 5-FU. Int J Cancer 1988; 41: 537–41
Thyss A, Milano G, Renée N, et al. Clinical pharmacokinetic study of 5-FU in continuous 5-day infusions for head and neck cancer. Cancer Chemother Pharmacol 1986; 16: 64–6
Gamelin EC, Danquechin-Dorval EM, Dumesnil YF, et al. Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer 1996; 77: 441–51
Decatris MP, Sundar S, O’Byrne KJ. Platinum-based chemotherapy in metastatic breast cancer: current status. Cancer Treat Rev 2004; 30: 53–81
Terret C, Erdociain E, Guimbaud R, et al. Dose and time dependencies of 5-fluorouracil pharmacokinetics. Clin Pharmacol Ther 2000; 68: 270–9
Jiang H, Lu J, Jiang J, et al. Important role of the dihydrouracil/uracil ratio in marked interpatient variations of fluoropyrimidine pharmacokinetics and pharmacodynamics. J Clin Pharmacol 2004; 44: 1260–72
Jodrell DI, Stewart M, Aird R, et al. 5-fluorouracil steady state pharmacokinetics and outcome in patients receiving protracted venous infusion for advanced colorectal cancer. Br J Cancer 2001; 84: 600–3
Findlay MP, Raynaud F, Cunningham D, et al. Measurement of plasma 5-fluorouracil by high-performance liquid chromatography with comparison of results to tissue drug levels observed using in vivo 19F magnetic resonance spectroscopy in patients on a protracted venous infusion with or without interferonalpha. Ann Oncol 1996; 7: 47–53
Port RE, Daniel B, Ding RW, et al. Relative importance of dose, body surface area, sex, and age for 5-fluorouracil clearance. Oncology 1991; 48: 277–81
Milano G, Etienne MC, Cassuto-Viguier E, et al. Influence of sex and age on fluorouracil clearance. J Clin Oncol 1992; 10: 1171–5
Lu Z, Zhang R, Diasio RB. Population characteristics of hepatic dihydropyrimidine dehydrogenase activity, a key metabolic enzyme in 5-fluorouracil chemotherapy. Clin Pharacol Ther 1995; 58: 512–22
Sloan JA, Goldberg RM, Sargent DJ, et al. Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol 2002; 20: 1491–8
Etienne MC, Chatelut E, Pivot X, et al. Co-variables influencing 5-fluorouracil clearance during continuous venous infusion: a NONMEM analysis. Eur J Cancer 1998; 34: 92–7
Fleming RA, Milano GA, Etienne MC, et al. No effect of dose, hepatic function, or nutritional status on 5-FU clearance following continuous (5-days), 5-FU infusion. Br J Cancer 1992; 66: 668–72
Maring JG, Piersma H, van Dalen A, et al. Extensive hepatic replacement due to liver metastases has no effect on 5-fluorouracil pharmacokinetics. Cancer Chemother Pharmacol 2003; 51: 167–73
Fleming GF, Schilsky RL, Schumm LP, et al. Phase I and pharmacokinetic study of 24-hour infusion 5-fluorouracil and leucovorin in patients with organ dysfunction. Ann Oncol 2003; 14: 1142–7
Rengelshausen J, Hull WE, Schwenger V, et al. Pharmacokinetics of 5-fluorouracil and its catabolites detemined by 19F nuclear magnetic resonance spectroscopy for a patient on chronic hemodialysis. Am J Kidney Dis 2002; 39: 1–7
Gusella M, Rebeschini M, Cartei G, et al. Effect of hemodialysis on the metabolic clearance of 5-fluorouracil in a patient with end-stage renal failure. Ther Drug Monit 2005; 27: 816–8
Bernadou J, Armand JP, Lopez A, et al. Complete urinary excretion profile of 5-fluorouracil during a six-day chemotherapeutic schedule, as resolved by 19F nuclear magnetic resonance. Clin Chem 1985; 31: 846–8
Milano G, Etienne MC. Potential importance of dihydropyrimidine dehydrogenase (DPD) in cancer chemotherapy. Pharmacogenetics 1994; 4: 301–6
Wei X, McLeod HL, McMurroug JL, et al. Molecular basis of the human dihydropyrimidine dehydrogenase deficiency and 5-fluorouracil toxicity. J Clin Invest 1996; 3: 610–5
Gonzalez FJ, Fernandez-Salguero P. Diagnosis analysis, clinical importance and molecular basis of dihydropyrimidine dehydrogenase deficiency. Trend Pharmacol Sci 1995; 16: 325–7
Etienne MC, Lagrange JL, Dassoville O, et al. Population study of dihydropyrimidine dehydrogenase in cancer patients. J Clin Oncol 1994; 12: 2248–53
Houyau P, Gay C, Chatelut E, et al. Severe fluorouracil toxicity in a patient with dihydropyrimidine dehydrogenase deficiency. J Natl Cancer Inst 1993; 85: 1602–3
Harris B, Carpenter JT, Diasio RB. Severe 5-fluorouracil toxicity secondary to dihydropyrimidine dehydrogenase deficiency. Cancer 1991; 68: 499–501
Lyss AP, Lilenbaum RC, Harris BE, et al. Severe 5-fluorouracil toxicity in a patient with decreased dihydropyrimidine dehydrogenase activity. Cancer Invest 1993; 11: 239–40
Diasio RB, Beavers TL, Carpenter JT. Familial deficiency of dihydropyrimidine dehydrogenase: biochemical basis for familial pyrimidinemia and severe 5-fluorouracil-induced toxicity. J Clin Invest 1988; 81: 47–51
Diasio RB, Lu Z. Dihydropyrimidine dehydrogenase activity and fluorouracil chemotherapy. J Clin Oncol 1994; 12: 2239–42
Heggie GD, Sommadossi JP, Cross DS, et al. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res 1987; 16: 2203–6
Jiang H, Lu J, Ji J. Circadian rhythm of dihydrouracil/uracil ratios in biological fluids: a potential biomarker for dihydropyrimidine dehydrogenase levels. Br J Pharmacol 2004; 141: 616–23
Harris BE, Song R, Soong SJ, et al. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res 1990; 50: 197–201
Fleming RA, Milano G, Thyss A, et al. Correlation between dihydropyrimidine dehydrogenase activity in peripheral mononuclear cells and systemic clearance of fluorouracil in cancer patients. Cancer Res 1992; 52: 2899–902
Grem JL, Yee LK, Venzon DJ, et al. Inter- and intraindividual variation in dihydropyrimidine dehydrogenase activity in peripheral blood mononuclear cells. Cancer Chemother Pharmacol 1997; 40: 117–25
Langouët AM, Metzger G, Comisso M, et al. Plasma concentration of 5-fluorouracil and mononuclear cell dihydropyrimidine dehydrogenase activity in patients treated with different chronomodulated schedules [abstract no. 135]. Biol Rhythm Res 1995; 26: 409
Tuchman M, von Roemeling R, Lanning RM, et al. Sources of variability of dihydropyrimidine dehydrogenase activity in human blood mononuclear cells. Annu Rev Chronopharmacol 1988; 5: 399–402
Van Kuilenburg AB, Poorter RL, Peters GL, et al. No circadian variation of dihydropyrimidine dehydrogenase, uridine phosphorylase, beta-alanine, and 5-fluorouracil during continuous infusion of 5-fluorouracil. Adv Exp Med Biol 1998; 431: 811–6
Barrat-Petit MA, Naulin-Ifi C, Mahler P, et al. Dihydropyrimidine deshydrogenase (DPD): rhythm and consequences. Pathol Biol (Paris) 2005; 53: 261–4
Lévi FA, Zidani R, Vannetzel JM, et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst 1994; 86: 1608–17
Lévi F, Zidani R, Misset JL, et al. Randomised muticentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. Lancet 1997; 350: 681–6
Metzger G, Massari C, Etienne MC, et al. Spontaneous or imposed circadian changes in plasma concentrations of 5-fluorouracil coadministered with folinic acid and oxaliplatin: relationship with mucosal toxicity in patients with cancer. Clin Pharmacol Ther 1994; 56: 190–201
Merkel U, Wedding U, Roskos M, et al. Pharmacokinetics of oxaliplatin during chronomodulated infusion in metastatic gastrointestinal cancer patients: a pilot investigation with preliminary results. Exp Toxicol Pathol 2003; 54: 475–9
Cattel L, La Grotta G, Infante L, et al. Pharmacokinetic study of oxaliplatin iv chronomodulated infusion combined with 5-fluorouracil iv continuous infusion in the treatment of advanced colorectal cancer. Farmaco 2003; 58: 1333–8
Joel SP, Papamichael D, Richards F, et al. Lack of pharmacokinetic interaction between 5-fluorouracil and oxaliplatin. Clin Pharmacol Ther 2004; 76: 45–54
Santini J, Milano G, Thyss A, et al. 5-FU therapeutic monitoring with dose adjustment leads to an improved therapeutic index in head and neck cancer. Br J Cancer 1989; 59: 287–90
Gamelin E, Boisdron-Celle M, Delva R, et al. Long-term weekly treatment of colorectal metastatic cancer with fluorouracil and leucovorin: results of a multicentric prospective trial of fluorouracil dosage optimization by pharmacokinetic monitoring in 152 patients. J Clin Oncol 1998; 16: 1470–8
Yoshida T, Araki E, Iigo M, et al. Clinical significance of monitoring serum levels of 5-fluorouracil by continuous infusion in patients with advanced colonic cancer. Cancer Chemother Pharmacol 1990; 26: 352–4
Milano G, Etienne MC, Renée N, et al. Relationship between fluorouracil systemic exposure and tumor response and patient survival. J Clin Oncol 1994; 12: 1291–5
Hillcoat BL, McCulloch PB, Figueredo AT, et al. Clinical response and plasma levels of 5-fluorouracil in patients with colonic cancer treated by drug infusion. Br J Cancer 1978; 38: 719–24
Di Paolo A, Danesi R, Falcone A, et al. Relationship between 5-fluorouracil disposition, toxicity, and dihydropyrimidine dehydrogenase activity in cancer patients. Ann Oncol 2001; 12: 1301–6
Di Paolo A, Ibrahim T, Danesi R, et al. Relationship between plasma concentrations of 5-fluorouracil and 5-fluoro-5,6-dihydrouracil and toxicity of 5-fluorouracil infusion in cancer patients. Ther Drug Monit 2002; 24: 588–93
Ychou M, Duffour J, Kramar A, et al. Individual 5-FU dose adaptation in metastatic colorectal cancer: results of a phase II study using a bimonthly pharmacokinetically intensified LV5FU2 regimen. Cancer Chemother Pharmacol 2003; 52: 282–90
Moore MJ, Bunting P, Yuan S, et al. Development and validation of a limited sampling strategy for 5-fluorouracil given by bolus intravenous administration. Ther Drug Monit 1993; 15: 394–9
Port RE, Edler L, Herrmann R, et al. Pharmacokinetics of 5-fluorouracil after short systemic infusion: plasma level at the end of the distribution phase as an indicator of the total area under the plasma concentration-time curve. Ther Drug Monit 1991; 13: 96–102
Gusella M, Ferrazzi E, Ferrari M, et al. New limited sampling strategy for determining 5-fluorouracil area under the concentration-time curve after rapid intravenous bolus. Ther Drug Monit 2002; 24: 425–31
Di Paolo A, Danesi R, Vannozzi F, et al. Limited sampling model for the analysis of 5-fluorouracil pharmacokinetics in adjuvant chemotherapy for colorectal cancer. Clin Pharmacol Ther 2002; 72: 627–37
Gamelin E, Jacob J, Danquechin-Dorval E, et al. Multicentric randomized trial comparing in weekly treatment of advanced colorectal cancer (CRC) intensified 5-fluorouracil and folinic acid (FA) with 5-FU pharmacokinetic monitoring to a constant dose calculated with body surface area [abstract no. 1039]. Annual Meeting of American Society of Clinical Oncology; 1998; Alexandria (VA)
Fety R, Rolland F, Barberi-Heyob M, et al. Clinical impact of pharmacokinetically-guided dose adaptation of 5-fluorouracil: results from a multicentric randomized trial in patients with locally advanced head and neck carcinomas. Clin Cancer Res 1998; 4: 2039–45
Fety R, Rolland F, Barberi-Heyob M, et al. Clinical randomized study of 5FU monitoring versus standard dose in patients with head and neck cancer: preliminary results. Anticancer Res 1994; 14: 2347–52
Diasio RB. The role of dihydropyrimidine dehydrogenase (DPD) modulation in 5-FU pharmacology. Oncology 1998; 12 Suppl. 7: 23–7
Omura K. Clinical implication of dihydropyrimidine dehydrogenase (DPD) activity in 5-FU-based chemotherapy: mutations in the DPD gene, and DPD inhibitory fluoropyrimidines. Int J Clin Oncol 2003; 8: 132–8
Milano G, Etienne MC. Individualizing therapy with 5-fluorouracil related to dihydropyrimidine dehydrogenase: theory and limits. Ther Drug Monit 1996; 18: 335–40
Maring JG, van Kuilenburg ABP, Haasjes J, et al. Reduced 5-FU clearance in a patient with low DPD activity due to heterozygosity for a mutant allele of the DPYD gene. Br J Cancer 2002; 86: 1028–33
Van Kuilenburg AB, Haasjes J, Richel DF, et al. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in DPD gene. Clin Cancer Res 2000; 6: 4705–12
Pullarkat ST, Stoehlmacher J, Ghaderi V, et al. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogenomics J 2001; 1: 65–70
Marsh S, McKay JA, Cassidy J, et al. Polymorphism in the thymidylate synthase promoter enhancer region in colorectal cancer. Int J Oncol 2001; 19: 383–6
Lecomte T, Ferraz JM, Zinzindohoue F, et al. Thymidylate synthase gene polymorphism predicts toxicity in colorectal cancer patients receiving 5-fluorouracil-based chemotherapy. Clin Cancer Res 2004; 10: 5880–8
Tomiak A, Vincent M, Earle CC, et al. Thymidylate synthase expression in stage II and III colon cancer: a retrospective review. Am J Clin Oncol 2001; 24: 597–602
Westra JL, Hollema H, Schaapveld M, et al. Predictive value of thymidylate synthase and dihydropyrimidine dehydrogenase protein expression on survival in adjuvantly treated stage III colon cancer patients. Ann Oncol 2005; 16: 1646–53
Wisotzkey JD, Toman J, Bell T, et al. MTHFR (C677T) polymorphisms and stage III colon cancer: response to therapy. Mol Diagn 1999; 4: 95–9
Etienne MC, Formento JL, Chazal M, et al. Methylenetetrahydrofolate reductase gene polymorphisms and response to fluorouracil-based treatment in advanced colorectal cancer patients. Pharmacogenetics 2004; 14: 785–92
Maring JG, Groen HJM, Wachters FM, et al. Genetic factors includencing pyrimidine-antagonist chemotherapy. Pharmacogenomics J 2005; 5: 226–43
Johnson MR, Wang K, Tillmanns S, et al. Structure organization of the human dihydropyrimidine dehydrogenase gene. Cancer Res 1997; 57: 1660–3
Wei X, Elizondo G, Sapone A, et al. Characterization of the human dihydropyrimidine dehydrogenase gene. Genomics 1998; 51: 391–400
Meinsma R, Fernandez-Salguero P, van Kuilenburg AB, et al. Human polymorphism in drug metabolism: mutation in the dihydropyrimidine dehydrogenase gene results in exon skipping and thymine uraciluria. DNA Cell Biol 1995; 14: 1–6
Yokota H, Fernandez-Salguero P, Furuya H, et al. cDNA cloning and chromosome mapping of human dihydropyrimidine dehydrogenase, an enzyme associated with 5-fluorouracil toxicity and congenital thymine uraciluria. J Biol Chem 1994; 269: 23192–6
Van Kuilenburg ABP. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluororuracil. Eur J Cancer 2004; 40: 939–50
Van Kuilenburg ABP, Vreken P, Abeling NGGM, et al. Genotype and phenotype in patients with dihydropyrimidine dehydrogenase deficiency. Hum Genet 1999; 104: 1–9
Van Kuilenburg ABP, Meinsma R, Zoetekouw L, et al. High prevalence of the IVS14 + 1G>A mutation in the dihydropyrimidine dehydrogenase gene of patients with severe 5-fluorouracil-associated toxicity. Pharmacogenetics 2002; 12: 555–8
Van Kuilenburg AB, Muller EW, Haasjes J, et al. Lethal outcome of a patient with a complete dihydropyrimidine dehydrogenase (DPD) deficiency after administration of 5-fluorouracil: frequency of the common IVS14 + 1G>A mutation causing DPD deficiency. Clin Cancer Res 2001; 7: 1149–53
Collie-Duguid ES, Etienne MC, Milano G, et al. Known variant DPYD alleles do not explain dihydropyrimidine dehydrogenase deficiency in cancer patients. Pharmacogenetics 2000; 10: 217–23
Vreken P, van Kuilenburg AB, Meinsma R, et al. Dihydropyrimidine dehydrogenase deficiency: a novel mutation and expression of missense mutations in E. coli, [published erratum appears in J Inherit Metab Dis 1998; 21: 623]. J Inherit Metab Dis 1998; 21: 276–9
Lazar A, Mau-Holzmann UA, Kolb H, et al. Multiple organ failure due to 5-fluorouracil chemotherapy in a patient with a rare dihydropyrimidine dehydrogenase gene variant. Onkologie 2004; 27: 559–62
Van Kuilenburg ABP, Baars JW, Meinsma R, et al. Lethal 5-fluorouracil toxicity associated with a novel mutation in the dihydropyrimidine dehydrogenase gene. Ann Oncol 2003; 14: 341–2
Kouwaki M, Hamajima N, Sumi S, et al. Identification of novel mutations in the dihydropyrimidine dehydrogenase gene in a Japanese patient with 5-fluorouracil toxicity. Clin Cancer Res 1998; 4: 2999–3004
Yamaguchi K, Arai Y, Kanda Y, et al. Germline mutation of dihydropyrimidine dehydrogenase gene among a Japanese population in relation to toxicity to 5-FU. Jpn J Cancer Res 2001; 92: 337–42
Vreken P, van Kuilenburg AB, Meinsma R, et al. Dihydropyrimidine dehydrogenase (DPD) deficiency: identification and expression of missense mutations C29R, R886H and R235W. Hum Genet 1997; 101: 333–8
Johnson MR, Wang K, Diasio RB. Profound dihydropyrimidine dehydrogenase deficiency resulting from a novel compound heterozygote genotype. Clin Cancer Res 2002; 8: 768–74
Van Kuilenburg ABP, Dobritzsch D, Meinsma R, et al. Novel disease-causing mutations in the dihydropyrimidine dehydrogenase gene interpreted by analysis of the three-dimensional protein structure. Biochem J 2002; 364: 157–63
Ezzeldin H, Johnson MR, Okamoto Y, et al. Denaturing high performance liquid chromatography analysis of the DPYD gene in patients with 5-fluorouracil toxicity. Clin Cancer Res 2003; 9: 3021–8
Vreken P, van Kuilenburg AB, Meinsma R, et al. Dihydropyrimidine dehydrogenase deficiency: identification of two novel mutations and expression of missense mutations in E. coli. Adv Exp Med Biol 1998; 431: 341–6
Van Kuilenburg AB, Meinsma R, Beke E, et al. Identification of three novel mutations in the dihydropyrimidine dehydrogenase gene associated with altered pre-mRNA splicing or protein function. Biol Chem 2005; 386: 319–24
Ogura K, Ohnuma T, Minamide Y, et al. Duhydropyrimidine dehydrogenase actibity in 150 healthy Japanese volunteers and identification of novel mutations. Clin Cancer Res 2005; 11: 5067–8
Seck K, Riemer S, Kates R, et al. Analysis of the DPYD gene implication in 5-fluorouracil catabolism in a cohort of Caucasian individuals. Clin Cancer Res 2005; 11: 5886–92
Ridge SA, Sludden J, Brown O, et al. Dihydropyrimidine dehydrogenase pharmacogenetics in Caucasian subjects. Br J Clin Pharmacol 1998; 46: 151–6
Ridge SA, Sludden J, Wei X, et al. Dihydropyrimidine dehydrogenase pharmacogenetics in patients with colorectal cancer. Br J Cancer 1998; 77: 497–500
Sumi S, Kidouchi K, Kondou M, et al. Possible prediction of adverse reactions to fluorouracil by the measurement of urinary dihydrothymine and thymine. Int J Mol Med 1998; 2: 477–82
Vreken P, Van Kuilenburg AB, Meinsma R, et al. Identification of novel point mutations in the dihydropyrimidine dehydrogenase gene. J Inherit Metab Dis 1997; 20: 335–8
Johnson MR, Hageboutros A, Wang K, et al. Life-threatening toxicity in a dihydropyrimidine dehydrogenase-deficient patient after treatment with topical 5-fluorouracil. Clin Cancer Res 1999; 5: 2006–11
Raida M, Schwabe W, Häusler P, et al. Prevalence of a common point mutation in the dihydropyrimidine dehydrogenase (DPD) gene within the 5′-splice donor site of intron 14 in patients with severe 5-fluorouracil (5-FU)-related toxicity compared with controls. Clin Cancer Res 2001; 7: 2832–9
Van Kuilenburg AB, Vreken P, Beex LV, et al. Heterozygosity for point mutation in an invariant splice donor site of dihydropyrimidine dehydrogenase and severe 5-fluorouracil related toxicity. Adv Exp Med Biol 1998; 431: 293–8
Van Kuilenburg BP, De Abreu RA, van Gennip AH. Pharmacogenetics and clinical aspects of dihydropyrimidine dehydrogenase deficiency. Ann Clin Biochem 2003; 40: 41–5
Kollmannsberger C, Bokemeyer C, Marx C, et al. Association between mutations in the dihydropyrimidine dehydrogenase gene and severe toxicity of treatment with 5-fluorouracil: a prospective multicenter study [abstract]. Onkologie 2001; 24 Suppl. 6: 101
Ezzeldin HH, Lee AM, Mattison LK, et al. Methylation of the DPYD promoter: an alternative mechanism for dihydropyrimidine dehydrogenase deficiency in cancer patients. Clin Cancer Res 2005; 11: 8699–705
Hori T, Takahashi E, Ayusawa D, et al. Regional assignment of the human thymidylate synthase (TS) gene to chromosome band 18p11.32 by nonisotopic in situ hybridization. Hum Genet 1990; 85: 576–80
Villafranca E, Okruzhnov Y, Dominguez MA, et al. Polymorphisms of the repeated sequences in the enhancer region of the thymidylate synthase gene promoter may predict downstaging after preoperative chemoradiation in rectal cancer. J Clin Oncol 2001; 19: 1779–86
Mandola MV, Stoehlmacher J, Muller-Weeks S, et al. A novel single nucleotide polymorphism within the 5′ tandem repeat polymorphism of the thymidylate synthase gene abolishes USF-1 binding and alters transcriptional activity. Cancer Res 2003; 63: 2898–904
Marcuello E, Altés A, del Rio E, et al. Single nucleotide polymorphism in the 5’ tandem repeat sequences of thymidylate synthase gene predicts for response to fluorouracil-based chemotherapy in advanced colorectal cancer patients. Int J Cancer 2004; 112: 733–7
Lu Z, Zhang R, Diasio RB. Dihydropyrimidine dehydrogenase activity in human peripheral blood mononuclear cells and liver: population characteristics newly identified deficient patients and clinical implication in 5-fluorouracil chemotherapy. Cancer Res 1993; 53: 5433–8
Johnson MR, Yan J, Shao L, et al. Semi-automated radioassay for determination of dihydropyrimidine dehydrogenase (DPD) activity: screening cancer patients for DPD deficiency, a condition associated with 5-fluorouracil toxicity. J Chromatogr B 1997; 696: 183–91
Tuchman M, Stoeckeler JS, Kiang DT, et al. Familial pyrimidinemia and pyrimidinuria associated with severe fluorouracil toxicity. N Engl J Med 1985; 313: 245–9
Johnson MR, Diasio RB. Importance of dihydropyrimidine dehydrogenase (DPD) deficiency in patients exhibiting toxicity following treatment with 5-fluorouracil. Adv Enzyme Regul 2001; 41: 151–7
Gamelin E, Boisdron-Celle M, Guérin-Meyer V, et al. Correlation between uracil and dihydrouracil plasma ratio, fluorouracil (5-FU) pharmacokinetic parameters, and tolerance in patients with advanced colorectal cancer: a potential interest for predicting 5-FU toxicity and determining optimal 5-FU dosage. J Clin Oncol 1999; 17: 1105–10
Sumi S, Kidouchi K, Hayashi K, et al. Urinary screening for pyrimidine metabolism disorders: reference ranges for dihydrouracil, uracil and dihydrouracil/uracil ratio. Adv Exp Med Biol 1998; 431: 191–5
Van Gennip AH, Abeling NG, Elzinga-Zoetekouw L, et al. Comparative study of thymine and uracil metabolism in healthy persons and in a patients with dihydropyrimidine dehydrogenase deficiency. Adv Exp Med Biol 1989; 253A: 111–8
Asai M, Sumi S, Kidouchi K, et al. Urinary pyrimidine analysis in healthy newborns, infants, children, adults and patients with congenital metabolic disease. Pediatr Int 2000; 42: 499–503
Hayashi K, Kidouchi K, Sumi S, et al. Possible prediction of adverse reactions to pyrimidine chemotherapy from urinary pyrimidine levels and a case of asymptomatic adult dihydropyrimidinuria. Clin Cancer Res 1996; 2: 1937–41
Bocci G, Danesi R, Di Paolo A, et al. Comparative pharmacokinetic analysis of 5-fluorouracil and its major metabolite 5-fluoro-5,6-dihydrouracil after conventional and reduced test dose in cancer patients. Clin Cancer Res 2000; 6: 3032–7
Mattison LK, Ezzeldin H, Carpenter M, et al. Rapid identification of dihydropyrimidine dehydrogenase deficiency by using a novel 2-l3C-uracil breath test. Clin Cancer Res 2004; 10: 2652–8
Acknowledgements
Su-arpa Ploylearmsaeng received a scholarship from the Köln-Fortune program of the Medical Faculty of the University of Cologne. No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ploylearmsaeng, Sa., Fuhr, U. & Jetter, A. How may Anticancer Chemotherapy with Fluorouracil be Individualised?. Clin Pharmacokinet 45, 567–592 (2006). https://doi.org/10.2165/00003088-200645060-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200645060-00002