Abstract
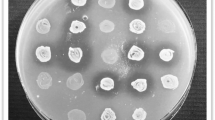
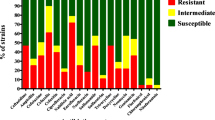
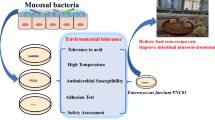
Avian pathogenic E. coli (APEC), an extra-intestinal pathogenic E. coli (ExPEC), causes colibacillosis in poultry and is also a potential foodborne zoonotic pathogen. Currently, APEC infections in poultry are controlled by antibiotic medication; however, the emergence of multi-drug-resistant APEC strains and increased restrictions on the use of antibiotics in food-producing animals necessitate the development of new antibiotic alternative therapies. Here, we tested the anti-APEC activity of multiple commensal and probiotic bacteria in an agar-well diffusion assay and identified Lacticaseibacillus rhamnosus GG and Bifidobacterium lactis Bb12 producing strong zone of inhibition against APEC. In co-culture assay, L. rhamnosus GG and B. lactis Bb12 completely inhibited the APEC growth by 24 h. Further investigation revealed that antibacterial product(s) in the culture supernatants of L. rhamnosus GG and B. lactis Bb12 were responsible for the anti-APEC activity. The analysis of culture supernatants using LC–MS/MS identified multiple novel bioactive peptides (VQAAQAGDTKPIEV, AFDNTDTSLDSTFKSA, VTDTSGKAGTTKISNV, and AESSDTNLVNAKAA) in addition to the production of lactic acid. The oral administration (108 CFU/chicken) of L. rhamnosus GG significantly (P < 0.001) reduced the colonization (~ 1.6 logs) of APEC in the cecum of chickens. Cecal microbiota analysis revealed that L. rhamnosus GG moderated the APEC-induced alterations of the microbial community in the cecum of chickens. Further, L. rhamnosus GG decreased (P < 0.05) the abundance of phylum Proteobacteria, particularly those belonging to Enterobacteriaceae (Escherichia-Shigella) family. These studies indicate that L. rhamnosus GG is a promising probiotic to control APEC infections in chickens. Further studies are needed to optimize the delivery of L. rhamnosus GG in feed or water and in conditions simulating the field to facilitate its development for commercial applications.







Similar content being viewed by others
Availability of Data and Material
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Lutful Kabir SM (2010) Avian colibacillosis and salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int J Environ Res Public Health 7(1):89–114. https://doi.org/10.3390/ijerph7010089
Kathayat D, Lokesh D, Ranjit S, Rajashekara G (2021) Avian pathogenic Escherichia coli (APEC): an overview of virulence and pathogenesis factors, zoonotic potential, and control strategies. Pathogens 10(4):467. https://doi.org/10.3390/pathogens10040467
Johnson TJ, Wannemuehler Y, Doetkott C, Johnson SJ, Rosenberger SC, Nolan LK (2008) Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J Clin Microbiol 46(12):3987–3996. https://doi.org/10.1128/jcm.00816-08
Mellata M (2013) Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog Dis 10(11):916–932. https://doi.org/10.1089/fpd.2013.1533
Liu CM, Stegger M, Aziz M, Johnson TJ, Waits K, Nordstrom L, Gauld L, Weaver B, Rolland D, Statham S, Horwinski J, Sariya S, Davis GS, Sokurenko E, Keim P, Johnson JR, Price LB (2018) Escherichia coli ST131-H22 as a foodborne uropathogen. MBio 9(4):e00470-e518. https://doi.org/10.1128/mBio.00470-18
Osman KM, Kappell AD, Elhadidy M, ElMougy F, El-Ghany WAA, Orabi A, Mubarak AS, Dawoud TM, Hemeg HA, Moussa IMI, Hessain AM, Yousef HMY (2018) Poultry hatcheries as potential reservoirs for antimicrobial-resistant Escherichia coli: a risk to public health and food safety. Sci Rep 8(1):5859. https://doi.org/10.1038/s41598-018-23962-7
Kathayat D, Helmy YA, Deblais L, Rajashekara G (2018) Novel small molecules affecting cell membrane as potential therapeutics for avian pathogenic Escherichia coli. Sci Rep 8(1):15329. https://doi.org/10.1038/s41598-018-33587-5
Dho-Moulin M, Fairbrother JM (1999) Avian pathogenic Escherichia coli (APEC). Vet Res 30(2–3):299–316
Nhung NT, Chansiripornchai N, Carrique-Mas JJ (2017) Antimicrobial resistance in bacterial poultry pathogens: a review. Fron Vet Sci 4:126. https://doi.org/10.3389/fvets.2017.00126
Gyles CL (2008) Antimicrobial resistance in selected bacteria from poultry. Anim Health Res Rev 9(2):149–158. https://doi.org/10.1017/S1466252308001552
Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Teillant A, Laxminarayan R (2015) Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA 112(18):5649–5654. https://doi.org/10.1073/pnas.1503141112
Hao H, Cheng G, Iqbal Z, Ai X, Hussain HI, Huang L, Dai M, Wang Y, Liu Z, Yuan Z (2014) Benefits and risks of antimicrobial use in food-producing animals. Front Microbiol 5:288. https://doi.org/10.3389/fmicb.2014.00288
Fijan S (2014) Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health 11(5):4745–4767. https://doi.org/10.3390/ijerph110504745
Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A (2012) Probiotic mechanisms of action. Ann Nutr Metab 61(2):160–174. https://doi.org/10.1159/000342079
Sánchez B, Delgado S, Blanco-Míguez A, Lourenço A, Gueimonde M, Margolles A (2017) Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res 61(1). https://doi.org/10.1002/mnfr.201600240
Servin AL (2004) Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28(4):405–440. https://doi.org/10.1016/j.femsre.2004.01.003
Ciorba MA (2012) A gastroenterologist’s guide to probiotics. Clin Gastroentero Hepatol 10(9):960–968. https://doi.org/10.1016/j.cgh.2012.03.024
Lievin-Le Moal V, Servin AL (2014) Anti-infective activities of Lactobacillus strains in the human intestinal microbiota: from probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin Microbiol Rev 27(2):167–199. https://doi.org/10.1128/cmr.00080-13
Vlasova AN, Kandasamy S, Chattha KS, Rajashekara G, Saif LJ (2016) Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet Immunol Immunopathol 172:72–84. https://doi.org/10.1016/j.vetimm.2016.01.003
Carter A, Adams M, La Ragione RM, Woodward MJ (2017) Colonisation of poultry by Salmonella Enteritidis S1400 is reduced by combined administration of Lactobacillus salivarius 59 and Enterococcus faecium PXN-33. Vet Microbiol 199:100–107. https://doi.org/10.1016/j.vetmic.2016.12.029
Van Coillie E, Goris J, Cleenwerck I, Grijspeerdt K, Botteldoorn N, Van Immerseel F, De Buck J, Vancanneyt M, Swings J, Herman L, Heyndrickx M (2007) Identification of lactobacilli isolated from the cloaca and vagina of laying hens and characterization for potential use as probiotics to control Salmonella Enteritidis. J Appl Microbiol 102(4):1095–1106. https://doi.org/10.1111/j.1365-2672.2006.03164.x
Saint-Cyr MJ, Haddad N, Taminiau B, Poezevara T, Quesne S, Amelot M, Daube G, Chemaly M, Dousset X, Guyard-Nicodeme M (2017) Use of the potential probiotic strain Lactobacillus salivarius SMXD51 to control Campylobacter jejuni in broilers. Int J Food Microbiol 247:9–17. https://doi.org/10.1016/j.ijfoodmicro.2016.07.003
Wang S, Peng Q, Jia HM, Zeng XF, Zhu JL, Hou CL, Liu XT, Yang FJ, Qiao SY (2017) Prevention of Escherichia coli infection in broiler chickens with Lactobacillus plantarum B1. Poult Sci 96(8):2576–2586. https://doi.org/10.3382/ps/pex061
Foltz KL, Ritzi MM, Barrett NW, Evans NP, Collins D, Sriranganathan N, Mahsoub H, Dalloul RA, Sewell J, Persia ME (2017) Efficacy of Lactobacillus plantarum supplementation in broilers challenged with avian pathogenic Escherichia coli and Salmonella Typhimurium. J Appl Poult Res 26(3):316–324. https://doi.org/10.3382/japr/pfw074
Ding S, Wang Y, Yan W, Li A, Jiang H, Fang J (2019) Effects of Lactobacillus plantarum 15–1 and fructooligosaccharides on the response of broilers to pathogenic Escherichia coli O78 challenge. PLoS One 14(6):e0212079. https://doi.org/10.1371/journal.pone.0212079
Forkus B, Ritter S, Vlysidis M, Geldart K, Kaznessis YN (2017) Antimicrobial probiotics reduce Salmonella enterica in turkey gastrointestinal tracts. Sci Rep 7:40695. https://doi.org/10.1038/srep40695
Mañes-Lázaro R, Van Diemen PM, Pin C, Mayer MJ, Stevens MP, Narbad A (2017) Administration of Lactobacillus johnsonii FI9785 to chickens affects colonisation by Campylobacter jejuni and the intestinal microbiota. Br Poult Sci 58(4):373–381. https://doi.org/10.1080/00071668.2017.1307322
Nakphaichit M, Sobanbua S, Siemuang S, Vongsangnak W, Nakayama J, Nitisinprasert S (2019) Protective effect of Lactobacillus reuteri KUB-AC5 against Salmonella Enteritidis challenge in chickens. Benef Microbes 10(1):43–54. https://doi.org/10.3920/bm2018.0034
Tabashsum Z, Peng M, Alvarado-Martinez Z, Aditya A, Bhatti J, Romo PB, Young A, Biswas D (2020) Competitive reduction of poultry-borne enteric bacterial pathogens in chicken gut with bioactive Lactobacillus casei. Sci Rep 10(1):16259. https://doi.org/10.1038/s41598-020-73316-5
Mangiamele P, Nicholson B, Wannemuehler Y, Seemann T, Logue CM, Li G, Tivendale KA, Nolan LK (2013) Complete genome sequence of the avian pathogenic Escherichia coli strain APEC O78. Genome Announc 1(2):e0002613. https://doi.org/10.1128/genomeA.00026-13
Fijan S (2016) Antimicrobial effect of probiotics against common pathogens. In: Rao V, Rao LG (eds) Probiotics and prebiotics in human nutrition and health. InTech, Rijeka, p Ch. 10. https://doi.org/10.5772/63141
De Keersmaecker SC, Verhoeven TL, Desair J, Marchal K, Vanderleyden J, Nagy I (2006) Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella Typhimurium is due to accumulation of lactic acid. FEMS Microbiol Lett 259(1):89–96. https://doi.org/10.1111/j.1574-6968.2006.00250.x
Fayol-Messaoudi D, Berger CN, Coconnier-Polter M-H, Liévin-Le Moal V, Servin AL (2005) pH-, lactic acid-, and non-lactic acid-dependent activities of probiotic lactobacilli against Salmonella enterica serovar Typhimurium. Appl Environ Microbiol 71(10):6008–6013. https://doi.org/10.1128/AEM.71.10.6008-6013
Xu Y, Zhao Z, Tong W, Ding Y, Liu B, Shi Y, Wang J, Sun S, Liu M, Wang Y, Qi Q, Xian M, Zhao G (2020) An acid-tolerance response system protecting exponentially growing Escherichia coli. Nat Commun 11(1):1496. https://doi.org/10.1038/s41467-020-15350-5
Shokryazdan P, Sieo CC, Kalavathy R, Liang JB, Alitheen NB, Faseleh Jahromi M, Ho YW (2014) Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. BioMed Res Int 2014:927268. https://doi.org/10.1155/2014/927268
Zhang Y, Zhang L, Du M, Yi H, Guo C, Tuo Y, Han X, Li J, Zhang L, Yang L (2011) Antimicrobial activity against Shigella sonnei and probiotic properties of wild lactobacilli from fermented food. Microbiol Res 167(1):27–31. https://doi.org/10.1016/j.micres.2011.02.006
Han J, Lin K, Sequeira C, Borchers CH (2015) An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal Chim Acta 854:86–94. https://doi.org/10.1016/j.aca.2014.11.015
Connor RI (2016) Bioactive molecules produced by probiotic bacteria and methods for isolating and using the same. United States trustees of Dartmouth College US20160024149A1
Helmy YA, Kassem II, Kumar A, Rajashekara G (2017) In vitro evaluation of the impact of the probiotic E. coli Nissle (1917) on Campylobacter jejuni’s invasion and intracellular survival in human colonic cells. Front Microbiol 8:1588. https://doi.org/10.3389/fmicb.2017.01588
He X, Zeng Q, Puthiyakunnon S, Zeng Z, Yang W, Qiu J, Du L, Boddu S, Wu T, Cai D, Huang S-H, Cao H (2017) Lactobacillus rhamnosus GG supernatant enhance neonatal resistance to systemic Escherichia coli K1 infection by accelerating development of intestinal defense. Sci Rep 7:43305. https://doi.org/10.1038/srep43305
Zhang L, Zhang L, Xa Z, Zeng X, Zhou L, Cao G, Ag C, Yang C (2016) Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J Anim Sci Biotechnol 7:3. https://doi.org/10.1186/s40104-016-0061-4
Dommels YEM, Kemperman RA, Zebregs YEMP, Draaisma RB, Jol A, Wolvers DAW, Vaughan EE, Albers R (2009) Survival of Lactobacillus reuteri DSM 17938 and Lactobacillus rhamnosus GG in the human gastrointestinal tract with daily consumption of a low-fat probiotic spread. Appl Environ Microbiol 75(19):6198–6204. https://doi.org/10.1128/AEM.01054-09
Deblais L, Helmy YA, Kathayat D, Huang H-c, Miller SA, Rajashekara G (2018) Novel imidazole and methoxybenzylamine growth inhibitors affecting Salmonella cell envelope integrity and its persistence in chickens. Sci Rep 8(1):13381. https://doi.org/10.1038/s41598-018-31249-0
Helmy YA, Kathayat D, Ghanem M, Jung K, Closs G Jr, Deblais L, Srivastava V, El-Gazzar M, Rajashekara G (2020) Identification and characterization of novel small molecule inhibitors to control Mycoplasma gallisepticum infection in chickens. Vet Microbiol 247:108799. https://doi.org/10.1016/j.vetmic.2020.108799
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu Y-X, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37(8):852–857. https://doi.org/10.1038/s41587-019-0209-9
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13(7):581–583. https://doi.org/10.1038/nmeth.3869
Yang G-Y, Yu J, Su J-H, Jiao L-G, Liu X, Zhu Y-H (2017) Oral administration of Lactobacillus rhamnosus GG ameliorates Salmonella infantis-induced inflammation in a pig model via activation of the IL-22BP/IL-22/STAT3 pathway. Front Cell Infect Microbiol 7:323. https://doi.org/10.3389/fcimb.2017.00323
Splichalova A, Jenistova V, Splichalova Z, Splichal I (2019) Colonization of preterm gnotobiotic piglets with probiotic Lactobacillus rhamnosus GG and its interference with Salmonella Typhimurium. Clin Exp Immunol 195(3):381–394. https://doi.org/10.1111/cei.13236
Liu J, Hu D, Chen Y, Huang H, Zhang H, Zhao J, Gu Z, Chen W (2018) Strain-specific properties of Lactobacillus plantarum for prevention of Salmonella infection. Food Funct 9(7):3673–3682. https://doi.org/10.1039/c8fo00365c
Yu J, Zhu Y-H, Yang G-Y, Zhang W, Zhou D, Su J-H, Wang J-F (2017) Anti-inflammatory capacity of Lactobacillus rhamnosus GG in monophasic variant Salmonella infected piglets is correlated with impeding NLRP6-mediated host inflammatory responses. Vet Microbiol 210:91–100. https://doi.org/10.1016/j.vetmic.2017.08.008
Khailova L, Frank DN, Dominguez JA, Wischmeyer PE (2013) Probiotic administration reduces mortality and improves intestinal epithelial homeostasis in experimental sepsis. Anesthesiology 119(1):166–177. https://doi.org/10.1097/ALN.0b013e318291c2fc
Sewaka M, Trullas C, Chotiko A, Rodkhum C, Chansue N, Boonanuntanasarn S, Pirarat N (2019) Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish Shellfish Immunol 86:260–268. https://doi.org/10.1016/j.fsi.2018.11.026
Zhang W, Zhu Y-H, Yang G-Y, Liu X, Xia B, Hu X, Su J-H, Wang J-F (2018) Lactobacillus rhamnosus GG affects microbiota and suppresses autophagy in the intestines of pigs ahallenged with Salmonella infantis. Front Microbiol 8:2705. https://doi.org/10.3389/fmicb.2017.02705
Chen L, Li H, Li J, Chen Y, Yang Y (2019) Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates microbiota dysbiosis in an experimental model of sepsis. Int J Mol Med 43(3):1139–1148. https://doi.org/10.3892/ijmm.2019.4050
Huang S-H, He L, Zhou Y, Wu C-H, Jong A (2009) Lactobacillus rhamnosus GG suppresses meningitic E. coli K1 penetration across human intestinal epithelial cells in vitro and protects neonatal rats against experimental hematogenous meningitis. Int J Microbiol 647862. https://doi.org/10.1155/2009/647862
Gao J, Li Y, Wan Y, Hu T, Liu L, Yang S, Gong Z, Zeng Q, Wei Y, Yang W, Zeng Z, He X, Huang S-H, Cao H (2019) A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front Microbiol 10:477. https://doi.org/10.3389/fmicb.2019.00477
Lu R, Fasano S, Madayiputhiya N, Morin NP, Nataro J, Fasano A (2009) Isolation, identification, and characterization of small bioactive peptides from Lactobacillus GG conditional media that exert both anti-Gram-negative and Gram-positive bactericidal activity. J Pediatr Gastroenterol Nutr 49(1):23–30. https://doi.org/10.1097/MPG.0b013e3181924d1e
Kathayat D, Closs G Jr, Helmy YA, Lokesh D, Ranjit S, Rajashekara G (2021) Peptides affecting outer membrane lipid asymmetry (MlaA-OmpC/F) system reduce avian pathogenic Escherichia coli (APEC) colonization in chickens. Appl Environ Microbiol 87(17):e0056721. https://doi.org/10.1128/aem.00567-21
Claes IJ, Schoofs G, Regulski K, Courtin P, Chapot-Chartier MP, Rolain T, Hols P, von Ossowski I, Reunanen J, de Vos WM, Palva A, Vanderleyden J, De Keersmaecker SC, Lebeer S (2012) Genetic and biochemical characterization of the cell wall hydrolase activity of the major secreted protein of Lactobacillus rhamnosus GG. PLoS One 7(2):e31588. https://doi.org/10.1371/journal.pone.0031588
Zanfardino A, Criscuolo G, Di Luccia B, Pizzo E, Ciavatta ML, Notomista E, Carpentieri A, Pezzella A, Varcamonti M (2017) Identification of a new small bioactive peptide from Lactobacillus gasseri supernatant. Benef Microbes 8(1):133–141. https://doi.org/10.3920/bm2016.0098
Mkrtchyan H, Gibbons S, Heidelberger S, Zloh M, Limaki HK (2010) Purification, characterisation and identification of acidocin LCHV, an antimicrobial peptide produced by Lactobacillus acidophilus n.v. Er 317/402 strain Narine. Int J Antimicrob Agents 35(3):255–260. https://doi.org/10.1016/j.ijantimicag.2009.11.017
Luz C, Saladino F, Luciano FB, Mañes J, Meca G (2017) In vitro antifungal activity of bioactive peptides produced by Lactobacillus plantarum against Aspergillus parasiticus and Penicillium expansum. LWT 81:128–135. https://doi.org/10.1016/j.lwt.2017.03.053
Diaz Carrasco JM, Casanova NA, Fernández Miyakawa ME (2019) Microbiota, gut health and chicken productivity: what is the connection? Microorganisms 7(10):374. https://doi.org/10.3390/microorganisms7100374
Chen L, Li H, Chen Y, Yang Y (2020) Probiotic Lactobacillus rhamnosus GG reduces mortality of septic mice by modulating gut microbiota composition and metabolic profiles. Nutr 78:110863. https://doi.org/10.1016/j.nut.2020.110863
Wang Y, Gong L, Wu Y-P, Cui Z-W, Wang Y-Q, Huang Y, Zhang X-P, Li W-F (2019) Oral administration of Lactobacillus rhamnosus GG to newborn piglets augments gut barrier function in pre-weaning piglets. J Zhejiang Univ Sci B 20(2):180–192. https://doi.org/10.1631/jzus.B1800022
Korpela K, Salonen A, Virta LJ, Kumpu M, Kekkonen RA, de Vos WM (2016) Lactobacillus rhamnosus GG intake modifies preschool children’s intestinal microbiota, alleviates penicillin-associated changes, and reduces antibiotic use. PLoS One 11(4):e0154012. https://doi.org/10.1371/journal.pone.0154012
Stanley D, Hughes RJ, Geier MS, Moore RJ (2016) Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: challenges presented for the identification of performance enhancing probiotic bacteria. Front Microbiol 7:187. https://doi.org/10.3389/fmicb.2016.00187
Khan S, Chousalkar KK (2020) Salmonella Typhimurium infection disrupts but continuous feeding of Bacillus based probiotic restores gut microbiota in infected hens. J Anim Sci Biotechnol 11:29. https://doi.org/10.1186/s40104-020-0433-7
Mohammedsaeed W, McBain AJ, Cruickshank SM, O’Neill CA (2014) Lactobacillus rhamnosus GG inhibits the toxic effects of Staphylococcus aureus on epidermal keratinocytes. Appl Environ Microbiol 80(18):5773–5781. https://doi.org/10.1128/AEM.00861-14
Kalaycı Yüksek F, Gümüş D, Gündoğan Gİ, Anğ Küçüker M (2020) Cell-free Lactobacillus sp supernatants modulate Staphylococcus aureus growth, adhesion and invasion to human osteoblast (HOB) cells. Curr Microbiol 78(1):125–132. https://doi.org/10.1007/s00284-020-02247-1
Šikić Pogačar M, Langerholc T, Mičetić-Turk D, Možina SS, Klančnik A (2020) Effect of Lactobacillus spp. on adhesion, invasion, and translocation of Campylobacter jejuni in chicken and pig small-intestinal epithelial cell lines. BMC Vet Res 16(1):34. https://doi.org/10.1186/s12917-020-2238-5
Hartmann M, Berditsch M, Hawecker J, Ardakani MF, Gerthsen D, Ulrich AS (2010) Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob Agents Chemother 54(8):3132–3142. https://doi.org/10.1128/AAC.00124-10
Acknowledgements
We thank Sochina Ranjit and Dhwani Parsana for technical assistance. We thank Wilbur Ouma for bioinformatics assistance. We thank MCIC and MS&P facilities, The Ohio State University, for analysis of samples for microbiome and LC-MS/MS studies. We thank Dr. Hanson, Megan Strother, Sara Tallmadge, and Ronna Wood for animal care and assistance with animal studies.
Funding
The research in Dr. Rajashekara laboratory was supported by the US Department of Agriculture National Institute for Food and Agriculture (USDA-NIFA) (Grant # 2015–68004-23131, 2020–6701-31401), The Ohio State University internal grants, and North Central Region Sustainable Research and Education (NCR-SARE) (Grant # GNC18-259).
Author information
Authors and Affiliations
Contributions
Conceptualization: GR, DK, and GC. Methodology: GR, DK, GC, YH, LD, and VS. Formal analysis and investigation: GR, DK, GC, YH, LD, and VS. Writing-original draft preparation: DK. Writing-review and editing: GR and DK. Funding acquisition: GR. Resources: GR. Supervision: GR.
Corresponding author
Ethics declarations
Ethics Approval
Animal study was approved by The Ohio State University Institutional Animal Care and Use Committee (IACUC, protocol # 2010A00000149).
Consent to Participate
Not applicable.
Consent for Publication
All authors read and approved the final manuscript.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kathayat, D., Closs, G., Helmy, Y.A. et al. In Vitro and In Vivo Evaluation of Lacticaseibacillus rhamnosus GG and Bifidobacterium lactis Bb12 Against Avian Pathogenic Escherichia coli and Identification of Novel Probiotic-Derived Bioactive Peptides. Probiotics & Antimicro. Prot. 14, 1012–1028 (2022). https://doi.org/10.1007/s12602-021-09840-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09840-1