Abstract
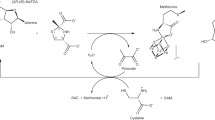
Thioredoxins are ubiquitous and conserved small proteins. The redox-active site is composed of highly conserved Cys32 and Cys35. In higher eukaryotes, thioredoxin evolved to a gain of function in nitrosative control, with 3 extra cysteines, Cys62, Cys69, and Cys73. Human thioredoxin 1 (hTrx) is directly involved in cellular signal transduction through S-nitrosation. The understanding of the mechanism of S-nitrosation is essential. Here we produced a mutant of hTrx containing only Cys62 (C62only). We report the almost full 1H, 15N, and 13C chemical shift assignment of the reduced and S-nitrosated C62only. This study will help to measure the reactivity Cys62 toward S-nitrosants and the stability of S-nitrosated Cys62.


Similar content being viewed by others
References
Barglow KT, Knutson CG, Wishnok JS et al (2011) Site-specific and redox-controlled S-nitrosation of thioredoxin. Proc Natl Acad Sci 108:E600–E606. https://doi.org/10.1073/pnas.1110736108
Cruzeiro-Silva C, Gomes-Neto F, Machado LESF et al (2014) Hydration and conformational equilibrium in yeast thioredoxin 1: implication for H + exchange. Biochemistry 53:2890–2902. https://doi.org/10.1021/bi401542v
Delaglio F, Grzesiek S, Vuister GW et al (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293. https://doi.org/10.1007/BF00197809
Fernando V, Zheng X, Walia Y et al (2019) S-nitrosylation: an emerging paradigm of redox signaling. Antioxidants 8:404. https://doi.org/10.3390/antiox8090404
Forman-Kay JD, Marius Clore G, Wingfield PT, Gronenborn AM (1991) High-resolution three-dimensional structure of reduced recombinant human thioredoxin in solution. Biochemistry 30:2685–2698. https://doi.org/10.1021/bi00224a017
Gal M, Edmonds KA, Milbradt AG et al (2011) Speeding up direct 15N detection: HCaN 2D NMR experiment. J Biomol NMR 51:497–504. https://doi.org/10.1007/s10858-011-9580-7
Grzesiek S, Bax A (1993) Amino acid type determination in the sequential assignment procedure of uniformly 13C/15N-enriched proteins. J Biomol NMR 3:185–204. https://doi.org/10.1007/BF00178261
Holmgren A (1995) Thioredoxin structure and mechanism: conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure 3:239–243. https://doi.org/10.1016/S0969-2126(01)00153-8
Ikura M, Kay LE, Bax A (1990) A novel approach for sequential assignment of 1H, 13C, and 15N spectra of larger proteins: heteronuclear triple-resonance three-dimensional NMR spectroscopy. Application to calmodulin. Biochemistry 29:4659–4667. https://doi.org/10.1021/bi00471a022
Iqbal A, Almeida FCL (2017) 1H, 13C and 15N chemical shift assignments of Saccharomyces cerevisiae type 1 thioredoxin in the oxidized state by solution NMR spectroscopy. Biomol NMR Assign 11:221–224. https://doi.org/10.1007/s12104-017-9752-9
Iqbal A, Gomes-Neto F, Myiamoto CA et al (2015a) Dissection of the water cavity of yeast thioredoxin 1: the effect of a hydrophobic residue in the cavity. Biochemistry 54:2429–2442. https://doi.org/10.1021/acs.biochem.5b00082
Iqbal A, Moraes AH, Valente AP, Almeida FCL (2015b) Structures of the reduced and oxidized state of the mutant D24A of yeast thioredoxin 1: insights into the mechanism for the closing of the water cavity. J Biomol NMR 63:417–423. https://doi.org/10.1007/s10858-015-9996-6
Kay LE, Xu GY, Singer AU et al (1993) A gradient-enhanced HCCH-TOCSY experiment for recording side-chain 1H and 13C correlations in H2O samples of proteins. J Magn Reson Ser B 101:333–337
Logan TM, Olejniczak ET, Xu RX, Fesik SW (1992) Side chain and backbone assignments in isotopically labeled proteins from two heteronuclear triple resonance experiments. FEBS Lett 314:413–418
Maciejewski MW, Schuyler AD, Gryk MR et al (2017) NMRbox: a resource for biomolecular NMR computation. Biophys J 112:1529–1534. https://doi.org/10.1016/j.bpj.2017.03.011
Mitchell DA, Marletta MA (2005) Thioredoxin catalyzes the s-nitrosation of the caspase-3 active site cysteine. Nat Chem Biol 1:154–158. https://doi.org/10.1038/nchembio720
Mitchell DA, Morton SU, Fernhoff NB, Marletta MA (2007) Thioredoxin is required for S-nitrosation of procaspase-3 and the inhibition of apoptosis in Jurkat cells. Proc Natl Acad Sci USA 104:11609–11614. https://doi.org/10.1073/pnas.0704898104
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Magn Reson Spectrosc 34:93–158
Talipov MR, Timerghazin QK (2013) Protein control of S-nitrosothiol reactivity: interplay of antagonistic resonance structures. J Phys Chem B 117:1827–1837. https://doi.org/10.1021/jp310664z
Vranken WF, Boucher W, Stevens TJ et al (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins Struct Funct Genet 59:687–696. https://doi.org/10.1002/prot.20449
Weichsel A, Brailey JL, Montfort WR (2007) Buried S-nitrosocysteine revealed in crystal structures of human thioredoxin. Biochemistry 46:1219–1227. https://doi.org/10.1021/bi061878r
Wittekind M, Mueller L (1993) HNCACB, a high-sensitivity 3D NMR experiment to correlate amide-proton and nitrogen resonances with the alpha- and beta-carbon resonances in proteins. J Magn Reson Ser B 101:201–205. https://doi.org/10.1006/jmrb.1993.1033
Wu C, Parrott AM, Fu C et al (2011) Thioredoxin 1-mediated post-translational modifications: reduction, transnitrosylation, denitrosylation, and related proteomics methodologies. Antioxidants Redox Signal 15:2565–2604. https://doi.org/10.1089/ars.2010.3831
Acknowledgements
This work was funded by FAPERJ Grants 239229 and 204432, awarded to FCLA, CNPq Grant 309564/2017-4, awarded to FCLA. We thank INBEB-INCT for funding. The assignments were deposited at the Biomagnetic Resonance Data Bank (BMRB ID 50485 and 50484. We also thank the Rural Federal University of Rio de Janeiro (UFRRJ) for the release of V. S. Almeida to attend the doctorate at the Federal University of Rio de Janeiro (UFRJ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almeida, V.S., Iqbal, A. & Almeida, F.C.L. 1H, 15N and 13C backbone and side‐chain assignments of reduced and S-nitrosated C62only mutant of human thioredoxin. Biomol NMR Assign 15, 261–265 (2021). https://doi.org/10.1007/s12104-021-10015-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-021-10015-w