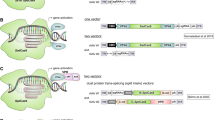
Abstract
As gene-editing technology has become more and more popular in the life sciences, CRISPR has brought good news to scientific researchers because of its efficiency, convenience, and wide application. Its wide application has also promoted the development of basic scientific research, agriculture, basic medicine, and clinical treatment. However, how the CRISPR/dCas9 system is effectively delivered to the target organs or cells is still unknown. This paper briefly introduces the CRISPR/dCas9 system and then lists some common delivery methods and their characteristics.

Similar content being viewed by others
References
Mojica, F. J. M., Ferrer, C., Juez, G., & Rodriguezvalera, F. (1995). Long stretches of short tandem repeats are present in the largest replicons of the archaea haloferax-mediterranei and haloferax-volcanii and could be involved in replicon partitioning. Molecular Microbiology.,17(1), 85–93.
Barrangou, R., Fremaux, C., Deveau, H., et al. (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science (New York, NY).,315, 1709–1712.
East-Seletsky, A., O’Connell, M. R., Knight, S. C., et al. (2016). Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature,538(7624), 270–273.
Abudayyeh, O. O., Gootenberg, J. S., Essletzbichler, P., et al. (2017). RNA targeting with CRISPR–Cas13. Nature,550(7675), 280–284.
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., & Charpentier, E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science,337(6096), 816–821.
Zhang, F., Wen, Y., & Guo, X. (2014). CRISPR/Cas9 for genome editing: progress, implications and challenges. Human Molecular Genetics,23, R40–R46.
Grissa, I., Vergnaud, G., & Pourcel, C. (2007). The CRISPRdb database and tools to display CRISPRs and to generate dictionaries of spacers and repeats. BMC Bioinformatics,8, 172.
Mussolino, C., & Cathomen, T. (2013). RNA guides genome engineering. Nature Biotechnology.,31, 208–209.
Lieber, M. R. (2010). The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annual Review of Biochemistry,79, 181–211.
Mojica, F. J. M., Díez-Villaseñor, C., García-Martínez, J., & Almendros, C. (2009). Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology,155(3), 733–740.
Mussolino, C., Mlambo, T., & Cathomen, T. (2015). Proven and novel strategies for efficient editing of the human genome. Current opinion in pharmacology.,24, 105–112.
Ran, F. A., Cong, L., Yan, W. X., et al. (2015). In vivo genome editing using Staphylococcus aureus Cas9. Nature,520(7546), 186–191.
Gilbert, L. A., Larson, M. H., Morsut, L., et al. (2013). CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell,154(2), 442–451.
Sander, J. D., & Joung, J. K. (2014). CRISPR-Cas systems for editing, regulating and targeting genomes. Nature Biotechnology,32(4), 347–355.
Cheng, A. W., Wang, H. Y., Yang, H., et al. (2013). Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Research,23(10), 1163–1171.
Maeder, M. L., Linder, S. J., Cascio, V. M., Fu, Y., Ho, Q. H., & Joung, J. K. (2013). CRISPR RNA-guided activation of endogenous human genes. Nature Methods,10(10), 977–979.
Gilbert Luke, A., Horlbeck Max, A., Adamson, B., et al. (2014). Genome-scale CRISPR-mediated control of gene repression and activation. Cell,159(3), 647–661.
Tanenbaum, M. E., Gilbert, L. A., Qi, L. S., Weissman, J. S., & Vale, R. D. (2014). A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell,159(3), 635–646.
Chavez, A., Scheiman, J., Vora, S., et al. (2015). Highly efficient Cas9-mediated transcriptional programming. Nature methods.,12(4), 326–328.
Konermann, S., Brigham, M. D., Trevino, A. E., et al. (2015). Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature,517(7536), 583–588.
Dominguez, A. A., Lim, W. A., & Qi, L. S. (2016). Beyond editing: Repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nature Reviews Molecular Cell Biology,17(1), 5–15.
Qi, L. S., Larson, M. H., Gilbert, L. A., et al. (2013). Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell,152(5), 1173–1183.
Piatek, A., Ali, Z., Baazim, H., et al. (2015). RNA-guided transcriptional regulation in planta via synthetic dCas9-based transcription factors. Plant Biotechnology Journal,13(4), 578–589.
Singh, A. K., Carette, X., Potluri, L.-P., et al. (2016). Investigating essential gene function in Mycobacterium tuberculosis using an efficient CRISPR interference system. Nucleic Acids Research,44(18), e143–e143.
Lino, C. A., Harper, J. C., Carney, J. P., & Timlin, J. A. (2018). Delivering CRISPR: A review of the challenges and approaches. Drug Deliv.,25(1), 1234–1257.
Horii, T., Arai, Y., Yamazaki, M., et al. (2014). Validation of microinjection methods for generating knockout mice by CRISPR/Cas-mediated genome engineering. Scientific Reports.,4, 4513.
Yang, H., Wang, H., Shivalila, C., Cheng, A., Shi, L., & Jaenisch, R. (2013). One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell,154(6), 1370–1379.
Hara, S., Tamano, M., Yamashita, S., et al. (2015). Generation of mutant mice via the CRISPR/Cas9 system using FokI-dCas9. Scientific Reports.,5(1), 11221.
Tröder, S. E., Ebert, L. K., Butt, L., Assenmacher, S., Schermer, B., & Zevnik, B. (2018). An optimized electroporation approach for efficient CRISPR/Cas9 genome editing in murine zygotes. PLoS ONE,13(5), e0196891–e0196891.
Li, S., Zhang, A., Xue, H., Li, D., & Liu, Y. (2017). One-step piggyBac transposon-based CRISPR/Cas9 activation of multiple genes. Molecular Therapy Nucleic Acids.,8, 64–76.
Saayman, S. M., Lazar, D. C., Scott, T. A., et al. (2016). Potent and targeted activation of latent HIV-1 using the CRISPR/dCas9 activator complex. Molecular Therapy,24(3), 488–498.
Suda, T., & Liu, D. (2015). Hydrodynamic delivery. Advances in Genetics,89, 89–111.
Zhou, H., Liu, J., Zhou, C., et al. (2018). In vivo simultaneous transcriptional activation of multiple genes in the brain using CRISPR–dCas9-activator transgenic mice. Nature Neuroscience,21(3), 440–446.
Li, L., He, Z. Y., Wei, X. W., Gao, G. P., & Wei, Y. Q. (2015). Challenges in CRISPR/CAS9 delivery: potential roles of nonviral vectors. Human Gene Therapy,26(7), 452–462.
Jost, M., Chen, Y., Gilbert, L. A., et al. (2017). Combined CRISPRi/a-based chemical genetic screens reveal that Rigosertib is a microtubule-destabilizing agent. Molecular Cell,68(1), 210–223.e216.
Hardcastle, N., Boulis, N. M., & Federici, T. (2018). AAV gene delivery to the spinal cord: Serotypes, methods, candidate diseases, and clinical trials. Expert Opinion on Biological Therapy.,18(3), 293–307.
Lykken, E. A., Shyng, C., Edwards, R. J., Rozenberg, A., & Gray, S. J. (2018). Recent progress and considerations for AAV gene therapies targeting the central nervous system. Journal of Neurodevelopmental Disorders.,10(1), 16.
Samulski, R. J., & Muzyczka, N. (2014). AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol.,1, 427–451.
Wu, Z. J., Yang, H. Y., & Colosi, P. (2010). Effect of genome size on AAV vector packaging. Molecular Therapy,18(1), 80–86.
Matharu, N., Rattanasopha, S., Tamura, S., et al. (2019). CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science,363(6424), eaau0629.
Nelson, C. E., Wu, Y., Gemberling, M. P., et al. (2019). Long-term evaluation of AAV-CRISPR genome editing for duchenne muscular dystrophy. Nature Medicine,25(3), 427–432.
Santiago-Fernández, O., Osorio, F. G., Quesada, V., et al. (2019). Development of a CRISPR/Cas9-based therapy for Hutchinson–Gilford progeria syndrome. Nature Medicine,25(3), 423–426.
Beyret, E., Liao, H.-K., Yamamoto, M., et al. (2019). Single-dose CRISPR–Cas9 therapy extends lifespan of mice with Hutchinson–Gilford progeria syndrome. Nature Medicine,25(3), 419–422.
Sun, J. Y., Anand-Jawa, V., Chatterjee, S., & Wong, K. K. (2003). Immune responses to adeno-associated virus and its recombinant vectors. Gene Therapy,10(11), 964–976.
Verma, R., Sahu, R., Singh, D. D., & Egbo, T. E. (2019). A CRISPR/Cas9 based polymeric nanoparticles to treat/inhibit microbial infections. Seminars in Cell & Developmental Biology,96, 44–52.
Liu, Q., Zhao, K., Wang, C., et al. (2018). Multistage delivery nanoparticle facilitates efficient CRISPR/dCas9 activation and tumor growth suppression in vivo. Advanced Science,6, 1801423.
Josipović, G., Tadić, V., Klasić, M., et al. (2019). Antagonistic and synergistic epigenetic modulation using orthologous CRISPR/dCas9-based modular system. Nucleic Acids Research,47(18), 9637–9657.
Weltner, J., Balboa, D., Katayama, S., et al. (2018). Human pluripotent reprogramming with CRISPR activators. Nature Communications,9(1), 2643–2643.
Li, Z., Zhang, D., Xiong, X., et al. (2017). A potent Cas9-derived gene activator for plant and mammalian cells. Nature Plants. https://doi.org/10.1038/s41477-017-0046-0.
Qiu, W., Xu, Z., Zhang, M., et al. (2019). Determination of local chromatin interactions using a combined CRISPR and peroxidase APEX2 system. Nucleic Acids Research,47(9), e52–e52.
Koo, B., Kim, D. E., Kweon, J., et al. (2018). CRISPR/dCas9-mediated biosensor for detection of tick-borne diseases. Sensor Actuators B-Chemical,273, 316–321.
Baumgart, M., Unthan, S., Kloß, R., et al. (2018). Corynebacterium glutamicum chassis C1*: Building and testing a novel platform host for synthetic biology and industrial biotechnology. ACS Synthetic Biology,7(1), 132–144.
Becker, J., Rohles, C. M., & Wittmann, C. (2018). Metabolically engineered Corynebacterium glutamicum for bio-based production of chemicals, fuels, materials, and healthcare products. Metabolic Engineering.,50, 122–141.
Izumi, Y., Chibata, I., & Itoh, T. (1978). Production and utilization of amino acids. Angewandte Chemie (International ed. in English),17(3), 176–183.
Cleto, S., Jensen, J. V., Wendisch, V. F., & Lu, T. K. (2016). Corynebacterium glutamicum metabolic engineering with CRISPR interference (CRISPRi). ACS Synthetic Biology,5(5), 375–385.
Yin, H., Song, C.-Q., Dorkin, J. R., et al. (2016). Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nature Biotechnology,34(3), 328–333.
Levy, J. M., Yeh, W.-H., Pendse, N., et al. (2020). Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nature Biomedical Engineering.,4(1), 97–110.
Makarova, K. S., Zhang, F., & Koonin, E. V. (2017). SnapShot: Class 2 CRISPR-Cas systems. Cell,168(1–2), 328–328.
Strecker, J., Jones, S., Koopal, B., et al. (2019). Engineering of CRISPR-Cas12b for human genome editing. Nature Communications,10(1), 212–212.
Doudna, J. A. (2020). The promise and challenge of therapeutic genome editing. Nature,578(7794), 229–236.
Cho, G. Y., Schaefer, K. A., Bassuk, A. G., Tsang, S. H., & Mahajan, V. B. (2018). CRISPR genome surgery in the retina in light of off-targeting. Retina,38(8), 1443–1455.
Acknowledgment
We thank the National Key Specialty Construction Project of Clinical Pharmacy (Grant No. 30305030698), the Science & Technology Program of Sichuan Province (Grant Nos. 2009SZ0226, 2014FZ0103, 2015JQO027, 2015ZR0160, 20ZDYF1490, and 20CXTD0043), the Health Department of Sichuan Province Grant Nos. 100491, 120111, and 17ZD038), Sichuan Cancer Hospital (Grant No. YB2019001), Chengdu City Science and Technology Project (Grant No. 11PPYB010SF-289), the Young Scholars Foundation of Sichuan Provincial People’s Hospital (Grant Nos. 30305030606 and 30305030859), the Cadre Health Care Research Project of Sichuan Province (Grant No. 2019-801), and Zambon Pharmaceutical Scientific Research Foundation of the Chengdu Pharmaceutical Association (Grant No. 201905).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.