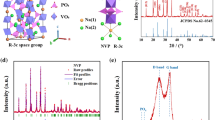
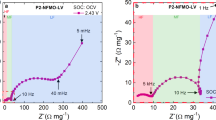
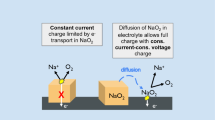
Abstract
We report here the heat generation and impedance characteristics of prototype 18650-sized sodium-ion cells using pristine Na3V2(PO4)3 (P-NVP) and modified Na3.2V1.8Zn0.2(PO4)3 (M-NVP) cathodes, hard carbon (HC) anode and an ether-based non-flammable electrolyte, 1 M NaBF4 in tetraglyme. Comparison of calorimetric studies performed on 18650-sized cells reveals lower heat generation in M-NVP versus HC compared to P-NVP versus HC owing to low internal resistance achieved as a result of Zn2+ doping in M-NVP. Both irreversible heat generation arose due to internal resistance and reversible heat generation caused by entropic changes in the electrode materials are elucidated. Furthermore, variation in subcomponents of internal resistance in both 18650-sized full cells and CR2016-sized half-cells is analysed by fitting electrochemical impedance spectra into equivalent circuit models. Individual contributions of anode and cathode to the impedance characteristics of the cells are determined by analysing impedance data of the half-cells using the distribution of relaxation times method. The results reveal lower diffusion resistance, as well as charge transfer resistance in M-NVP cells compared to P-NVP counterpart, accounting for the observed lower total internal resistance in M-NVP versus HC and thus lower heat generation in M-NVP versus HC cell than P-NVP versus HC cell.









Similar content being viewed by others
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study.
Abbreviations
- ∂U/∂T :
-
Entropic coefficient/V K−1
- C :
-
Capacitance/F
- C p :
-
Specific heat capacity/kJ kg−1 K−1
- \(\frac{{{\text{d}}T}}{{{\text{d}}t}}\) :
-
Temperature gradient/K s−1
- Est. :
-
Estimated
- I :
-
Current/A
- I p :
-
Pulse current/A
- Im:
-
Imaginary
- m :
-
Mass/kg
- Q 1, Q 2 :
-
Constant phase elements
- Q irr :
-
Irreversible heat generation/J
- Q rev :
-
Reversible heat generation/J
- Q total :
-
Total heat generation/J
- R :
-
Resistance/Ω
- R CEI :
-
Cathode–electrolyte interphase resistance/Ω
- R ct :
-
Charge-transfer resistance/Ω
- R d :
-
Diffusion resistance/Ω
- Re:
-
Real
- R i :
-
Internal resistance/Ω
- R s :
-
Series resistance/Ω
- R SEI :
-
Solid–electrolyte interphase resistance/Ω
- R total :
-
Total resistance/Ω
- T :
-
Instantaneous temperature/K
- τ :
-
Time constant/s
- V :
-
Cell voltage/V
- Z :
-
Impedance/Ω
- Δt :
-
Time step/s
References
Yabuuchi N, Kubota K, Dahbi M, Komaba S. Research development on sodium-ion batteries. Chem Rev. 2014;114:11636–82. https://doi.org/10.1021/cr500192f.
Kubota K, Komaba S. Review—practical issues and future perspective for na-ion batteries. J Electrochem Soc. 2015;162:A2538–50. https://doi.org/10.1149/2.0151514jes.
Yang H, Amiruddin S, Bang HJ, Sun YK, Prakash J. A review of li-ion cell chemistries and their potential use in hybrid electric vehicles. J Ind Eng Chem. 2006;12:12–38.
Spotnitz R, Franklin J. Abuse behavior of high-power, lithium-ion cells. J Power Sour. 2003;113:81–100.
Zhang J, Huang J, Li Z, Wu B, Nie Z, Sun Y, et al. Comparison and validation of methods for estimating heat generation rate of large-format lithium-ion batteries. J Therm Anal Calorim. 2014;117:447–61. https://doi.org/10.1007/s10973-014-3672-z.
Hong J-S, Maleki H, Al Hallaj S, Redey L, Selman JR. Electrochemical-calorimetric studies of lithium-ion cells. J Electrochem Soc. 1998;145:1489. https://doi.org/10.1149/1.1838509.
Bazinski SJ, Wang X. The influence of cell temperature on the entropic coefficient of a lithium iron phosphate (LFP) pouch cell. J Electrochem Soc. 2014;161:A168–75. https://doi.org/10.1149/2.082401jes.
Balasundaram M, Ramar V, Yap C, Li Lu, Tay AAO, Balaya P. Heat loss distribution: impedance and thermal loss analyses in LiFePO4/graphite 18650 electrochemical cell. J Power Sour. 2016;328:413–21.
Manikandan B, Yap C, Balaya P. Towards understanding heat generation characteristics of Li-ion batteries by calorimetry, impedance, and potentiometry studies. J Electrochem Soc. 2017;164:A2794–800. https://doi.org/10.1149/2.1811712jes.
Du K, Wang C, Subasinghe LU, Gajella SR, Law M, Rudola A, et al. A comprehensive study on the electrolyte, anode and cathode for developing commercial type non-flammable sodium-ion battery. Energy Storage Mater. 2020;29:287–99.
Wang Q, Zhao X, Ye J, Sun Q, Ping P, Sun J. Thermal response of lithium-ion battery during charging and discharging under adiabatic conditions. J Therm Anal Calorim. 2016;124:417–28. https://doi.org/10.1007/s10973-015-5100-4.
Archibald E, Kennedy R, Marr K, Jeevarajan J, Ezekoye O. Characterization of thermally induced runaway in pouch cells for propagation. Fire Technol. 2020;56:2467–90. https://doi.org/10.1007/s10694-020-00974-2.
Abraham DP, Roth EP, Kostecki R, McCarthy K, MacLaren S, Doughty DH. Diagnostic examination of thermally abused high-power lithium-ion cells. J Power Sour. 2006;161:648–57.
Al Hallaj S, Prakash J, Selman JR. Characterization of commercial Li-ion batteries using electrochemical–calorimetric measurements. J Power Sour. 2000;87:186–94.
Onda K, Kameyama H, Hanamoto T, Ito K. Experimental study on heat generation behavior of small lithium-ion secondary batteries. J Electrochem Soc. 2003;150:A285. https://doi.org/10.1149/1.1543947.
Yang H, Prakash J. Determination of the reversible and irreversible heats of a LiNi0.8Co0.15Al0.05O2/natural graphite cell using electrochemical-calorimetric technique. J Electrochem Soc. 2004;151:A1222. https://doi.org/10.1149/1.1765771.
Liu G, Ouyang M, Lu L, Li J, Han X. Analysis of the heat generation of lithium-ion battery during charging and discharging considering different influencing factors. J Therm Anal Calorim. 2014;116:1001–10. https://doi.org/10.1007/s10973-013-3599-9.
Schuster E, Ziebert C, Melcher A, Rohde M, Seifert HJ. Thermal behavior and electrochemical heat generation in a commercial 40 Ah lithium ion pouch cell. J Power Sour. 2015;286:580–9. https://doi.org/10.1016/j.jpowsour.2015.03.170.
Yang C, Xin S, Mai L, You Y. Materials design for high-safety sodium-ion battery. Adv Energy Mater. 2021;11:2000974. https://doi.org/10.1002/aenm.202000974.
Subasinghe LU, Gajella SR, Rudola A, Balaya P. Analysis of heat generation and impedance characteristics of Prussian blue analogue cathode-based 18650-type sodium-ion cells. J Electrochem Soc. 2020;167: 110504. https://doi.org/10.1149/1945-7111/ab9ee9.
Huang J, Albero Blanquer L, Bonefacino J, Logan ER, Alves Dalla Corte D, Delacourt C, et al. Operando decoding of chemical and thermal events in commercial Na(Li)-ion cells via optical sensors. Nat Energy. 2020;5:674–83. https://doi.org/10.1038/s41560-020-0665-y.
Zhao J, Zhao L, Chihara K, Okada S, Yamaki JI, Matsumoto S, et al. Electrochemical and thermal properties of hard carbon-type anodes for Na-ion batteries. J Power Sour. 2013;244:752–7. https://doi.org/10.1016/j.jpowsour.2013.06.109.
Rudola A, Du K, Balaya P. Monoclinic sodium iron hexacyanoferrate cathode and non-flammable glyme-based electrolyte for inexpensive sodium-ion batteries. J Electrochem Soc. 2017;164:A1098–109. https://doi.org/10.1149/2.0701706jes.
Zhao J, He J, Ding X, Zhou J, Ma Y, Wu S, et al. A novel sol–gel synthesis route to NaVPO4F as cathode material for hybrid lithium ion batteries. J Power Sour. 2010;195:6854–9.
Xia X, Dahn JR. NaCrO2 is a fundamentally safe positive electrode material for sodium-ion batteries with liquid electrolytes. Electrochem Solid-State Lett. 2012;15:A1. https://doi.org/10.1149/2.002201esl.
Ruffo R, Fathi R, Kim DJ, Jung YH, Mari CM, Kim DK. Impedance analysis of Na0.44MnO2 positive electrode for reversible sodium batteries in organic electrolyte. Electrochim Acta. 2013;108:575–82. https://doi.org/10.1016/j.electacta.2013.07.009.
Patra J, Huang H-T, Xue W, Wang C, Helal AS, Li J, et al. Moderately concentrated electrolyte improves solid–electrolyte interphase and sodium storage performance of hard carbon. Energy Storage Mater. 2019;16:146–54.
Kehne P, Guhl C, Ma Q, Tietz F, Alff L, Hausbrand R, et al. Sc-substituted Nasicon solid electrolyte for an all-solid-state NaxCoO2/Nasicon/Na sodium model battery with stable electrochemical performance. J Power Sour. 2019;409:86–93.
Zheng W, Gao R, Zhou T, Huang X. Enhanced electrochemical performance of Na3V2(PO4)3with Ni2+doping by a spray drying-assisted process for sodium ion batteries. Solid State Ionics. 2018;324:183–90. https://doi.org/10.1016/j.ssi.2018.07.006.
Terada S, Susa H, Tsuzuki S, Mandai T, Ueno K, Dokko K, et al. Glyme-sodium bis(fluorosulfonyl)amide complex electrolytes for sodium ion batteries. J Phys Chem C. 2018;122:16589–99. https://doi.org/10.1021/acs.jpcc.8b04367.
Law M, Ramar V, Balaya P. Synthesis, characterisation and enhanced electrochemical performance of nanostructured Na 2 FePO 4 F for sodium batteries. RSC Adv. 2015;5:50155–64.
Law M, Ramar V, Balaya P. Na2MnSiO4as an attractive high capacity cathode material for sodium-ion battery. J Power Sour. 2017;359:277–84. https://doi.org/10.1016/j.jpowsour.2017.05.069.
Law M, Balaya P. NaVPO4F with high cycling stability as a promising cathode for sodium-ion battery. Energy Storage Mater. 2018;10:102–13. https://doi.org/10.1016/j.ensm.2017.08.007.
Ratnakumar BV, Smart MC, Whitcanack LD, Ewell RC. The impedance characteristics of Mars Exploration Rover Li-ion batteries. J Power Sour. 2006;159:1428–39.
Onda K, Ohshima T, Nakayama M, Fukuda K, Araki T. Thermal behavior of small lithium-ion battery during rapid charge and discharge cycles. J Power Sour. 2006;158:535–42.
Jalkanen K, Aho T, Vuorilehto K. Entropy change effects on the thermal behavior of a LiFePO4/graphite lithium-ion cell at different states of charge. J Power Sour. 2013;243:354–60.
Wang L, Zhao J, He X, Gao J, Li J, Wan C, et al. Electrochemical impedance spectroscopy (EIS) Study of LiNi 1/3 Co 1/3 Mn 1/3 O 2 for Li-ion batteries. Int J Electrochem Sci. 2012;7:345–53.
Tröltzsch U, Kanoun O, Tränkler H-R. Characterizing aging effects of lithium ion batteries by impedance spectroscopy. Electrochim Acta. 2006;51:1664–72.
Schichlein H, Ivers-tiffe E, Ivers-tiffe E, Voigts M, Kru A, Schichlein H, et al. Deconvolution of electrochemical impedance spectra for the identification of electrode reaction mechanisms in solid oxide fuel cells. J Appl Electrochem. 2002;32:875–82. https://doi.org/10.1023/A:1020599525160.
Schmidt JP, Berg P, Schönleber M, Weber A, Ivers-Tiffée E. The distribution of relaxation times as basis for generalized time-domain models for Li-ion batteries. J Power Sour. 2013;221:70–7. https://doi.org/10.1016/j.jpowsour.2012.07.100.
Schmidt JP, Chrobak T, Ender M, Illig J, Klotz D, Ivers-Tiffée E. Studies on LiFePO4 as cathode material using impedance spectroscopy. J Power Sour. 2011;196:5342–8.
Manikandan B, Ramar V, Yap C, Balaya P. Investigation of physico-chemical processes in lithium-ion batteries by deconvolution of electrochemical impedance spectra. J Power Sour. 2017;361:300–9. https://doi.org/10.1016/j.jpowsour.2017.07.006.
Illig J, Schmidt JP, Weiss M, Weber A, Ivers-Tiffée E. Understanding the impedance spectrum of 18650 LiFePO4-cells. J Power Sour. 2013;239:670–9.
Wan TH, Saccoccio M, Chen C, Ciucci F. Influence of the discretization methods on the distribution of relaxation times deconvolution: implementing radial basis functions with DRTtools. Electrochim Acta. 2015;184:483–99. https://doi.org/10.1016/j.electacta.2015.09.097.
Laidler KJ. The development of the Arrhenius equation. J Chem Educ. 1984;61:494. https://doi.org/10.1021/ed061p494.
Lu L, Han X, Li J, Hua J, Ouyang M. A review on the key issues for lithium-ion battery management in electric vehicles. J Power Sour. 2013;226:272–88. https://doi.org/10.1016/j.jpowsour.2012.10.060.
Saw LH, Ye Y, Tay AAO. Electrochemical-thermal analysis of 18650 Lithium Iron Phosphate cell. Energy Convers Manag. 2013;75:162–74. https://doi.org/10.1016/j.enconman.2013.05.040.
Lu W, Yang H, Prakash J. Determination of the reversible and irreversible heats of LiNi0.8Co0.2O2/mesocarbon microbead Li-ion cell reactions using isothermal microcalorimetery. Electrochim Acta. 2006;51:1322–9.
Hou H, Qiu X, Wei W, Zhang Y, Ji X. Carbon anode materials for advanced sodium-ion batteries. Adv Energy Mater. 2017;7:1602898. https://doi.org/10.1002/aenm.201602898.
Zhang X, Rui X, Chen D, Tan H, Yang D, Huang S, et al. Na 3 V 2 (PO 4) 3: an advanced cathode for sodium-ion batteries. Nanoscale. 2019;11:2556–76.
Renganathan S, Sikha G, Santhanagopalan S, White RE. Theoretical analysis of stresses in a lithium ion cell. J Electrochem Soc. 2010;157:A155. https://doi.org/10.1149/1.3261809.
Linden D, Reddy TB. Handbook of batteries. 3rd ed. McGraw-Hill Companies Inc.; 2001.
Dou J, Kang X, Wumaier T, Hua N, Han Y, Xu G. Oxalic acid-assisted preparation of LiFePO4/C cathode material for lithium-ion batteries. J Solid State Electrochem. 2012;16:1925–31. https://doi.org/10.1007/s10008-011-1585-3.
Ramar V, Balaya P. Enhancing the electrochemical kinetics of high voltage olivine LiMnPO4 by isovalent co-doping. Phys Chem Chem Phys. 2013;15:17240.
Li H, Yu X, Bai Y, Wu F, Wu C, Liu L, et al. Effects of Mg doping on the remarkably enhanced electrochemical performance of Na3V2(PO4)3 cathode materials for sodium ion batteries. J Mater Chem A. 2015;3:9578–86.
Li H, Bai Y, Wu F, Ni Q, Wu C. Na-Rich Na 3+ x V 2–x Ni x (PO 4) 3 /C for sodium ion batteries: controlling the doping site and improving the electrochemical performances. ACS Appl Mater Interfaces. 2016;8:27779–87. https://doi.org/10.1021/acsami.6b09898.
Nizamov FA, Togulev PN, Abdullin DR, Khantimerov SM, Balaya P, Suleimanov NM. Antisite defects and valence state of vanadium in Na3V2(PO4)3. Phys Solid State. 2016;58:475–80. https://doi.org/10.1134/S1063783416030240.
Schweiger H-G, Obeidi O, Komesker O, Raschke A, Schiemann M, Zehner C, et al. Comparison of several methods for determining the internal resistance of lithium ion cells. Sensors. 2010;10:5604–25.
Väli R, Jänes A, Lust E. Alkali-metal insertion processes on nanospheric hard carbon electrodes: an electrochemical impedance spectroscopy study. J Electrochem Soc. 2017;164:E3429–37. https://doi.org/10.1149/2.0431711jes.
Xiao B, Soto FA, Gu M, Han KS, Song J, Wang H, et al. Lithium-pretreated hard carbon as high-performance sodium-ion battery anodes. Adv Energy Mater. 2018;8:1801441. https://doi.org/10.1002/aenm.201801441.
Shaju KM, Subba Rao GV, Chowdari BVR. Influence of li-ion kinetics in the cathodic performance of layered Li(Ni1/3Co1/3Mn1/3)O2. J Electrochem Soc. 2004;151:A1324. https://doi.org/10.1149/1.1775218.
Zaban A, Aurbach D. Impedance spectroscopy of lithium and nickel electrodes in propylene carbonate solutions of different lithium salts A comparative study. J Power Sour. 1995;54:289–95.
Qiu S, Xiao L, Sushko ML, Han KS, Shao Y, Yan M, et al. Manipulating adsorption-insertion mechanisms in nanostructured carbon materials for high-efficiency sodium ion storage. Adv Energy Mater. 2017;7:1700403. https://doi.org/10.1002/aenm.201700403.
Wang K, Jin Y, Sun S, Huang Y, Peng J, Luo J, et al. Low-cost and high-performance hard carbon anode materials for sodium-ion batteries. ACS Omega. 2017;2:1687–95. https://doi.org/10.1021/acsomega.7b00259.
Gavrilova TP, Khantimerov SM, Fatykhov RR, Yatsyk IV, Cherosov MA, Lee HS, et al. Magnetic properties and vanadium oxidation state in α-Li3V2(PO4)3/C composite: magnetization and ESR measurements. Solid State Commun. 2021;323:114108.
Acknowledgements
The authors thank the National Research Foundation (under the Energy Programme and administrated by Energy Market Authority), the Ministry of Education in Singapore and the National University of Singapore for funding. LUS thanks the Ministry of Education (MoE) in Singapore for research scholarship and extends his gratitude to Kang Du and Abhinav Tripathi for the support and guidance given.
Funding
This research is supported by National Research Foundation, under the Energy Programme and administrated by Energy Market Authority (EP Award No. NRF2015EWT-EIRP002-017/WBS No. R-265-000-568-279), Ministry of Education in Singapore (WBS No. R-265-000-510-112) and National University of Singapore (WBS No. R-261-510-001-646).
Author information
Authors and Affiliations
Contributions
LUS performed conceptualization, methodology, formal analysis, investigation, visualization, and writing—original draft. CW was involved in methodology, investigation, validation, and supplying 18,650 cells for the study. SRG contributed to methodology, investigation, validation, and resources related to 18,650 cell fabrication, funding acquisition—project schedule and budget planning, and writing—review and editing. ML done conceptualization, methodology, and investigation related to cathode material, and writing—review and editing. BM performed conceptualization, methodology, and resources related to thermal characterization, and writing—review and editing. PB contributed to conceptualization, resources, supervision, project administration, funding acquisition, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Subasinghe, L.U., Wang, C., Gajjela, S.R. et al. A study on heat generation characteristics of Na3V2(PO4)3 cathode and hard carbon anode-based sodium-ion cells. J Therm Anal Calorim 147, 8631–8649 (2022). https://doi.org/10.1007/s10973-021-11151-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11151-0