Abstract
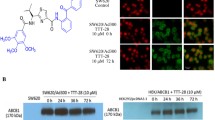
Taxane agents are of particular interest in non-small cell lung carcinomas (NSCLC) treatment, while multidrug resistance (MDR) mediated by P-glycoprotein (P-gp) limits their clinical efficacy. TM2, a chemically semi-synthesized taxane derivative, exerted significant anti-cancer efficacy in vitro and in vivo, especially against vincristine-resistant and adriamycin-resistant cancer cells. In this study, the anti-cancer effect of TM2 on drug-resistant NSCLC was evaluated both in vitro and in vivo, and the mechanism underlying its anti-MDR activity was further clarified. It was found that TM2 was significantly cytotoxic to cisplatin- and paclitaxel-resistant A549 (human non-small cell lung cancer) cells that overexpressing P-gp, resulting in IC50 values of 0.19 µM and 0.12 µM. TM2 micelles (5 mg/kg, 10 mg/kg, 20 mg/kg, i.v., 21 days) inhibited the growth of MDR xenograft with the maximal inhibitory rate up to 80.4%. Moreover, TM2 caused cell cycle arrest in the G2-M phase and apoptosis in drug-resistant cells through promoting tubulin polymerization, which acted in a way similar to taxane agents. Notably, TM2 acted as a P-gp inhibitor with high binding affinity, which resulted in impaired efflux function through forming H-bonds and ATP hydrolysis to induce P-gp conformational alterations. These findings indicated that TM2 displays anti-MDR activity with the potential for the treatment of NSCLC, which can inhibit P-gp function and stabilize microtubule polymerization.










Similar content being viewed by others
References
Abel B, Tosh DK, Durell SR, Murakami M, Vahedi S, Jacobson KA, Ambudkar SV (2019) Evidence for the Interaction of A3 Adenosine Receptor Agonists at the Drug-Binding Site(s) of Human P-glycoprotein (ABCB1). Mol Pharmacol 96:180–192. doi:https://doi.org/10.1124/mol.118.115295
Abolhoda A, Wilson AE, Ross H, Danenberg PV, Burt M, Scotto KW (1999) Rapid activation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin cancer research: official J Am Association Cancer Res 5:3352–3356doi
Al-Katib AM, Smith MR, Kamanda WS, Pettit GR, Hamdan M, Mohamed AN, Chelladurai B, Mohammad RM (1998) Bryostatin 1 down-regulates mdr1 and potentiates vincristine cytotoxicity in diffuse large cell lymphoma xenografts. Clin cancer research: official J Am Association Cancer Res 4:1305–1314doi
Alam A, Kowal J, Broude E, Roninson I, Locher KP (2019) Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. Science 363:753–756. doi:https://doi.org/10.1126/science.aav7102
Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G (2009) Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323:1718–1722. doi:https://doi.org/10.1126/science.1168750
Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM (1999) Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39:361–398. doi:https://doi.org/10.1146/annurev.pharmtox.39.1.361
Bai Z, Gao M, Zhang H, Guan Q, Xu J, Li Y, Qi H, Li Z, Zuo D, Zhang W, Wu Y (2017) BZML, a novel colchicine binding site inhibitor, overcomes multidrug resistance in A549/Taxol cells by inhibiting P-gp function and inducing mitotic catastrophe. Cancer Lett 402:81–92. doi:https://doi.org/10.1016/j.canlet.2017.05.016
Chen CJ, Chin JE, Ueda K, Clark DP, Pastan I, Gottesman MM, Roninson IB (1986) Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell 47:381–389. doi:https://doi.org/10.1016/0092-8674(86)90595-7
Chiang NJ, Lin CI, Liou JP, Kuo CC, Chang CY, Chen LT, Chang JY (2013) A novel synthetic microtubule inhibitor, MPT0B214 exhibits antitumor activity in human tumor cells through mitochondria-dependent intrinsic pathway. PLoS ONE 8:e58953. doi:https://doi.org/10.1371/journal.pone.0058953
Duran GE, Derdau V, Weitz D, Philippe N, Blankenstein J, Atzrodt J, Semiond D, Gianolio DA, Mace S, Sikic BI (2018) Cabazitaxel is more active than first-generation taxanes in ABCB1(+) cell lines due to its reduced affinity for P-glycoprotein. Cancer Chemother Pharmacol 81:1095–1103. doi:https://doi.org/10.1007/s00280-018-3572-1
Globisch C, Pajeva IK, Wiese M (2006) Structure-activity relationships of a series of tariquidar analogs as multidrug resistance modulators. Bioorg Med Chem 14:1588–1598. doi:https://doi.org/10.1016/j.bmc.2005.10.058
Gut I, Ojima I, Vaclavikova R, Simek P, Horsky S, Linhart I, Soucek P, Kondrova E, Kuznetsova LV, Chen J (2006) Metabolism of new-generation taxanes in human, pig, minipig and rat liver microsomes. Xenobiotica 36:772–792. doi:https://doi.org/10.1080/00498250600829220
Hanna N, Johnson D, Temin S, Baker S Jr, Brahmer J, Ellis PM, Giaccone G, Hesketh PJ, Jaiyesimi I, Leighl NB, Riely GJ, Schiller JH, Schneider BJ, Smith TJ, Tashbar J, Biermann WA, Masters G (2017) Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin oncology: official J Am Soc Clin Oncol 35:3484–3515. doi:https://doi.org/10.1200/JCO.2017.74.6065
Holland IB, Blight MA (1999) ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J Mol Biol 293:381–399. doi:https://doi.org/10.1006/jmbi.1999.2993
Kastan MB, Bartek J (2004) Cell-cycle checkpoints and cancer. Nature 432:316–323. doi:https://doi.org/10.1038/nature03097
Krishna R, Mayer LD (2000) Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci 11:265–283. doi:https://doi.org/10.1016/s0928-0987(00)00114-7
Kunihara M, Nagai J, Murakami T, Takano M (1998) Renal excretion of rhodamine 123, a P-glycoprotein substrate, in rats with glycerol-induced acute renal failure. J Pharm Pharmacol 50:1161–1165. doi:https://doi.org/10.1111/j.2042-7158.1998.tb03328.x
Kurdziel KA, Kiesewetter DO, Carson RE, Eckelman WC, Herscovitch P (2003) Biodistribution, radiation dose estimates, and in vivo Pgp modulation studies of 18F-paclitaxel in nonhuman primates. J Nucl Med 44:1330–1339doi
Li Y, Geng M, Wang J, Liu H, Ding N, Zhang W (2011) in PNT p 30, CN.doi
Lombard AP, Liu C, Armstrong CM, Cucchiara V, Gu X, Lou W, Evans CP, Gao AC (2017) ABCB1 Mediates Cabazitaxel-Docetaxel Cross-Resistance in Advanced Prostate Cancer. Mol Cancer Ther 16:2257–2266. doi:https://doi.org/10.1158/1535-7163.MCT-17-0179
Loo TW, Clarke DM (1995) Membrane topology of a cysteine-less mutant of human P-glycoprotein. J Biol Chem 270:843–848. doi:https://doi.org/10.1074/jbc.270.2.843
Loo TW, Clarke DM (2000) The packing of the transmembrane segments of human multidrug resistance P-glycoprotein is revealed by disulfide cross-linking analysis. J Biol Chem 275:5253–5256. doi:https://doi.org/10.1074/jbc.275.8.5253
Loo TW, Clarke DM (2005) Do drug substrates enter the common drug-binding pocket of P-glycoprotein through “gates”? Biochem Biophys Res Commun 329:419–422. doi:https://doi.org/10.1016/j.bbrc.2005.01.134
Maloney SM, Hoover CA, Morejon-Lasso LV, Prosperi JR (2020) Mechanisms of Taxane Resistance. Cancers (Basel)12.doi:https://doi.org/10.3390/cancers12113323
Milosevic Z, Bankovic J, Dinic J, Tsimplouli C, Sereti E, Dragoj M, Paunovic V, Milovanovic Z, Stepanovic M, Tanic N, Dimas K, Pesic M (2018) Potential of the dual mTOR kinase inhibitor AZD2014 to overcome paclitaxel resistance in anaplastic thyroid carcinoma. Cell Oncol (Dordr) 41:409–426. doi:https://doi.org/10.1007/s13402-018-0380-x
Nobili S, Landini I, Mazzei T, Mini E (2012) Overcoming tumor multidrug resistance using drugs able to evade P-glycoprotein or to exploit its expression. Med Res Rev 32:1220–1262. doi:https://doi.org/10.1002/med.20239
Ojima I, Das M (2009) Recent advances in the chemistry and biology of new generation taxoids. J Nat Prod 72:554–565. doi:https://doi.org/10.1021/np8006556
Podolski-Renic A, Andelkovic T, Bankovic J, Tanic N, Ruzdijic S, Pesic M (2011) The role of paclitaxel in the development and treatment of multidrug resistant cancer cell lines. Biomed Pharmacother 65:345–353. doi:https://doi.org/10.1016/j.biopha.2011.04.015
Seelig A, Landwojtowicz E (2000) Structure-activity relationship of P-glycoprotein substrates and modifiers. Eur J Pharm Sci 12:31–40. doi:https://doi.org/10.1016/s0928-0987(00)00177-9
Subhani S, Jayaraman A, Jamil K (2015) Homology modelling and molecular docking of MDR1 with chemotherapeutic agents in non-small cell lung cancer. Biomed Pharmacother 71:37–45. doi:https://doi.org/10.1016/j.biopha.2015.02.009
Sun Y, Bao X, Ren Y, Jia L, Zou S, Han J, Zhao M, Han M, Li H, Hua Q, Fang Y, Yang J, Wu C, Chen G, Wang L (2019) Targeting HDAC/OAZ1 axis with a novel inhibitor effectively reverses cisplatin resistance in non-small cell lung cancer. Cell Death Dis 10:400doi. https://doi.org/10.1038/s41419-019-1597-y
Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5:219–234. doi:https://doi.org/10.1038/nrd1984
Wang L, Li H, Ren Y, Zou S, Fang W, Jiang X, Jia L, Li M, Liu X, Yuan X, Chen G, Yang J, Wu C (2016) Targeting HDAC with a novel inhibitor effectively reverses paclitaxel resistance in non-small cell lung cancer via multiple mechanisms. Cell death & disease7:e2063.doi:https://doi.org/10.1038/cddis.2015.328
Wang Q, Liu Y, Pu C, Zhang H, Tan X, Gou J, He H, Yin T, Zhang Y, Wang Y, Tang X (2018) Drug-Polymer Interaction, Pharmacokinetics and Antitumor Effect of PEG-PLA/Taxane Derivative TM2 Micelles for Intravenous Drug Delivery. Pharm Res 35:208. doi:https://doi.org/10.1007/s11095-018-2477-3
Wang NN, Wang LH, Li Y, Fu SY, Xue X, Jia LN, Yuan XZ, Wang YT, Tang X, Yang JY, Wu CF (2018) Targeting ALDH2 with disulfiram/copper reverses the resistance of cancer cells to microtubule inhibitors. Exp Cell Res 362:72–82. doi:https://doi.org/10.1016/j.yexcr.2017.11.004
Yared JA, Tkaczuk KH (2012) Update on taxane development: new analogs and new formulations. Drug Des Devel Ther 6:371–384. doi:https://doi.org/10.2147/DDDT.S28997
Zhang JW, Ge GB, Liu Y, Wang LM, Liu XB, Zhang YY, Li W, He YQ, Wang ZT, Sun J, Xiao HB, Yang L (2008) Taxane’s substituents at C3’ affect its regioselective metabolism: different in vitro metabolism of cephalomannine and paclitaxel. Drug Metab Dispos 36:418–426. doi:https://doi.org/10.1124/dmd.107.018242
Zhang Y, Liu Y, Wang N, Liu H, Gou J, He H, Zhang Y, Yin T, Wang Y, Tang X (2021) Preparation of mPEG-b-PLA/TM2 Micelle Lyophilized Products by Mixed Lyoprotectors and Antitumor Effect In Vivo. AAPS PharmSciTech 22:38. doi:https://doi.org/10.1208/s12249-020-01885-9
Acknowledgments and funding
This work was supported by the National Natural Science Foundation of China (No. 81903112, 81973365, 81773780).
Author information
Authors and Affiliations
Contributions
Concept and design: Lina Jia. Acquisition of data: Xiaoyun Gao, Yi Fang, Qixiang Hua. Analysis and interpretation: Haotian Zhang. Original Draft: Lina Jia and Lihui Wang. Review and editing: Jingyu Yang and Chunfu Wu.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The animal study followed the Guideline for Animal Experimentation of Shenyang Pharmaceutical University. This study was approved by the Institute Ethical Committee for the experimental Use of Animals in Shenyang Pharmaceutical University (Permit Number: 20181022). All procedures adhered to principles expressed in the Declaration of Helsinki.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, L., Gao, X., Fang, Y. et al. TM2, a novel semi-synthetic taxoid, exerts anti-MDR activity in NSCLC by inhibiting P-gp function and stabilizing microtubule polymerization. Apoptosis 27, 1015–1030 (2022). https://doi.org/10.1007/s10495-022-01767-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-022-01767-4