Abstract
The classic BCR-ABL1-negative myeloproliferative neoplasm (MPN) is a highly heterogeneous hematologic tumor that includes three subtypes, namely polycythemia vera (PV), essential thrombocytosis (ET), and primary myelofibrosis (PMF). Despite having the same JAK2V617F mutation, the clinical manifestations of these three subtypes of MPN differ significantly, which suggests that the bone marrow (BM) immune microenvironment may also play an important role. In recent years, several studies have shown that peripheral blood monocytes play an important role in promoting MPN. However, to date, the role of BM monocytes/macrophages in MPN and their transcriptomic alterations remain incompletely understood. The purpose of this study was to clarify the role of BM monocytes/macrophages in MPN patients with the JAK2V617F mutation. MPN patients with the JAK2V617F mutation were enrolled in this study. We investigated the roles of monocytes/macrophages in the BM of MPN patients, using flow cytometry, monocyte/macrophage enrichment sorting, cytospins and Giemsa-Wright staining, and RNA-seq. Pearson correlation coefficient analysis was also used to detect the correlation between BM monocytes/macrophages and the MPN phenotype. In the present study, the proportion of CD163+ monocytes/macrophages increased significantly in all three subtypes of MPN. Interestingly, the percentages of CD163+ monocytes/macrophages are positively correlated with HGB in PV patients and PLT in ET patients. In contrast, the percentages of CD163+ monocytes/macrophages are negatively correlated with HGB and PLT in PMF patients. It was also found that CD14+CD16+ monocytes/macrophages increased and correlated with MPN clinical phenotypes. RNA-seq analyses demonstrated that the transcriptional expressions of monocytes/macrophages in MPN patients are relatively distinct. Gene expression profiles of BM monocytes/macrophages suggest a specialized function in support of megakaryopoiesis in ET patients. In contrast, BM monocytes/macrophages yielded a heterogeneous status in the support or inhibition of erythropoiesis. Significantly, BM monocytes/macrophages shaped an inflammatory microenvironment, which, in turn, promotes myelofibrosis. Thus, we characterized the roles of increased monocytes/macrophages in the occurrence and progression of MPNs. Our findings of the comprehensive transcriptomic characterization of BM monocytes/macrophages provide important resources to serve as a basis for future studies and future targets for the treatment of MPN patients.
Similar content being viewed by others
Introduction
Myeloproliferative neoplasms (MPNs) comprise a group of chronic and heterogenous hematological malignancies that exhibit terminal myeloid cell expansion, including erythrocytes, thrombocytes, and leukocytes [1,2,3]. The three main Philadelphia chromosome–negative MPN subentities, polycythemia vera (PV), essential thrombocytosis (ET), and primary myelofibrosis (PMF), are characterized by chronic activation of the JAK-STAT pathway, resulting from transforming mutations in JAK2, calreticulin, or MPL genes [3,4,5,6,7,8]. Genetic analysis of patients with MPNs revealed that more than 95% of patients with PV and ∼50% of patients with ET or PMF carry an activating point mutation in the JAK2 gene (JAK2V617F) [9,10,11,12,13]. It is necessary to note that, despite the presence of the same JAK2V617F mutation, the clinical presentation of patients with different types of MPN varies considerably, which indicates that other “driver factors” also play an important role in MPNs.
The bone marrow (BM) niche is a complex and dynamic structure composed of a multitude of cell types which functionally create an interactive network that facilitates hematopoietic stem cell (HSC) development and maintenance [14,15,16,17]. Monocytes/macrophages are the key component of the BM microenvironment, which have been reported to support the development of erythroid cells [18,19,20], HSCs [21, 22], and megakaryocytes [23] in normal conditions. It has also been reported that macrophages promote erythroid cell [18, 24] and platelet production [23] in mouse models. In addition, Marina Dorigatti Borges et al. found that PB monocytes expressed several higher molecules, including cell adhesion, iron metabolism, and those capable of identifying and engulfing damaged and senescent erythrocytes, which suggests that monocytes may be more likely to attach erythroid cells and could, therefore, contribute to form erythroblastic islands (EBIs) if differentiated from macrophages [25]. In contrast, macrophages, which have been shown to induce the proliferation of myofibroblasts via vitamin D receptor signaling [26]], have been found to be increased in BM biopsies of PMF patients [27]. These studies demonstrate that monocytes/macrophages play distinct roles in MPNs; however, the roles of BM monocytes/macrophages in human MPN patients remain unclear.
Although experiments and clinical studies have revealed that the JAK2V617F mutation is associated with MPNs, the transcriptional consequences of the JAK2V617F mutation in different cellular components of the BM have not yet been fully elucidated. Using ScRNA-seq, Tong et al. report that HSCs with the JAK2V617F mutation in ET show strong megakaryocyte (Mk) lineage priming, are more sensitive to IFN signaling than to Mk differentiation, and exhibit distinct signatures upon treatment [3]. Van Egeren et al. found the same results of JAK2V617F mutant HSCs [28]. In addition, they also found that monocytes with JAK2V617F have a pro-inflammatory intermediate monocyte phenotype, and JAK2V617F monocytes express SLAMF7, which is associated with fibrosis in MPNs [28]. Maekawa et al. also report that SLAMF7high monocytes increased in the PB of patients with MF in correlation with the JAK2V617F mutation [4], and anti-SLAMF7 antibodies suppressed monocyte-derived fibrocyte differentiation and could be a potent therapeutic agent for MF [4]. Therefore, an understanding of the mechanism that drives activated monocytes/macrophages in MPN will guide strategies to target monocytes/macrophages in this disease.
In this study, we aimed to investigate the role of monocytes/macrophages in MPN patients with the JAK2V617F mutations. We performed flow cytometry to examine the expression of CD163, CD14, and CD16 in monocytes/macrophages from JAK2V617F mutant MPNs and present RNA-seq analysis of BM monocytes/macrophages of four healthy controls (HCs, also known as normal bone marrow, NBM), four PV patients, three ET patients, and five PMF patients. The analysis in our study shows that either CD163 or combined CD14 and CD16 are significantly elevated in MPN and correlates closely with the clinical phenotype of MPNs. RNA-seq analysis of BM monocytes/macrophages of four HCs, four PV patients, three ET patients, and five PMF patients demonstrated that different subtypes of MPN have relatively specific BM immune microenvironments. And the comprehensive transcriptomic characterization of BM monocytes/macrophages provides important resources to serve as a basis for future studies and future targets for the treatment of MPN patients.
Materials and methods
Patients and bone marrow samples
Eighty-five PV patients, 131 ET patients, and 42 PMF patients with the JAK2V617F mutation who were treated at the First Affiliated Hospital of Zhengzhou University between January 14, 2017, and March 05, 2021, were enrolled in this study for the analysis of peripheral blood routine and BM aspiration biopsy. An amount of 3–5 ml BM was aspirated from four HCs, four PV patients, three ET patients and five PMF patients. This study was approved by the Ethics Committee at the First Affiliated Hospital of Zhengzhou University. All methods and procedures associated with this study were conducted in accordance with the Good Clinical Practice guidelines and the ethical principles of the Declaration of Helsinki, as well as the local laws.
Preparation of single cell mononuclear cells and enrichment of CD163+ monocytes/macrophages
The BM of MPN patients were collected and processed for single-cell preparation for flow cytometry and enrichment of CD163+ monocytes/macrophages. The single cell suspensions were prepared as previously described [18, 29]. Then, BM CD163+ monocytes/macrophages from HCs and MPN patients were enriched with the CD163 MicroBead Kit (Miltenyi, Cat#:130–124-420), according to the manufacturer's instructions. BM cells were first incubated with CD163-biotin for 15 min on ice in the dark. The cells were then washed with PBS plus 2% FBS and 2 mM EDTA, centrifuged at 1200 rpm for 10 min, and then incubated with anti-biotin microbeads for 15 min on ice in the dark. The cells were again washed with PBS plus 2% FBS and 2 mM EDTA, again centrifuged at 300 g for 10 min, and then resuspended with 2-mL PBS plus 2% FBS and 2 mM EDTA. Finally, the BM CD163+ monocytes/macrophages were enriched with Quadro MACS, according to the manufacturer’s instructions.
Flow cytometry staining and analyses
Flow cytometry staining and analyses were performed as previous described [18, 29,30,31]. Briefly, 5 × 106 mononuclear cells (MNCs) were blocked with 50-μL human Fc receptor blocking (dilution 1:100) for 15 min at 4 °C, then stained with APC-CD163, Percp-CD14, and PE-CY7-CD16 for 30 min on ice in the dark. After staining, the cells were washed once with PBS plus 0.5% BSA and 2 mM EDTA. DAPI was used to gate out dead cells. Then the cells were resuspended with PBS plus 0.5% BSA and 2 mM EDTA, and run on a BD Air III (BD bioscience). Flow Jo software (BD) was used to analyze the data.
RNA-sequencing and analysis
RNA-seq was prepared and analyzed as previously described [29, 31,32,33,34]. RNA was extracted from the monocytes/macrophages of the control and MPN patients. Approximately 100 ng of total RNA was used as input for the cDNA library preparation, which was preformed using an Illumina TruSeq kit followed by sequencing with an Illumina HiSeq 4000 platform (Beijing Genomics Institute, BGI, China). Gene read counts for Gencode hg19 version 31 protein coding genes were generated using Kallisto for the analysis of the RNA-seq data. Differential gene expressions were examined with DESeq2, using a Wald test with a 0.05 adjusted p value cutoff and independent filtering to remove genes with lower expression levels, as previously described [29, 31,32,33,34]. The KEGG signaling pathway was performed as previously described [29, 31,32,33,34].
Cytospins and Giemsa-Wright staining
Cytospins and Giemsa-Wright staining of monocytes/macrophages from the control and MPN patients were performed as described in our previous papers [18, 29, 31].
Statistics
GraphPad Prism 9.0 software (GraphPad Software, Inc.) was used to perform the statistical analysis. All the experiments were repeated at least three times. All data were reported as mean ± SEM. Comparisons between different groups were performed by the student’s t test. Pearson correlation coefficient analysis was used to detect the correlations between monocytes/macrophages CD163 and CD14 combined with CD16 with the MPN phenotype. P < 0.05 was selected to indicate a statistically significant difference.
Results
The proportion of BM CD163+ or CD14+CD16+ monocytes/macrophages increased in MPN and correlated with different MPN subtypes
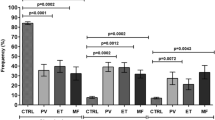
To evaluate the hypothesis that CD163+ monocytes/macrophages play significant roles in MPN, we collected the BM samples of MPN patients with the JAK2V617F mutation. We first confirmed that phenotype of distinct MPN using blood routine and BM biopsies (Supplementary Fig. 1A and B). Then, we examined the percentage of BM CD163+ monocytes/macrophages in the subtypes of MPN. Figure 1A demonstrates the representative flow cytometry image of CD163 in PV, ET, PMF, and HCs. A quantitative analysis indicated that the percentage of BM CD163+ monocytes/macrophages significantly increase in MPNs (Fig. 1B). Furthermore, the BM CD163+ monocytes/macrophages populations in HC and in MPNs were morphologically different (Fig. 1C). Next, we analyzed the correlation of the BM CD163+ monocyte/macrophage percentage with clinical phenotypes of MPNs. Figure 1D indicates that the BM CD163+ monocyte/macrophage percentage is positively correlated with the HGB levels in PV patients. Figure 1E shows that BM CD163+ monocyte/macrophage percentage is positively correlated with PLT in ET patients. In contrast, BM CD163+ monocyte/macrophage percentage is negatively associated with HGB in PMF patients (Fig. 1F). CD14+CD16+ monocytes/macrophages display an inflammatory function [30, 35, 36]. We next performed flow cytometry to analyze the CD14+CD16+ subset in CD163+ monocytes/macrophages in MPNs. Similarly, the percentage of the CD14+CD16+ subpopulation was also found to increase in PV, ET and PMF as compared to HCs (Supplementary Fig. 2A and B). The BM CD14+CD16+ subpopulation percentage is positively correlated with HGB in PV patients (Supplementary Fig. 2C), but is negatively correlated with HGB in PMF patients (Supplementary Fig. 2E), while there is no correlation between the CD14+CD16+ subpopulation percentage and PLT in ET patients (Supplementary Fig. 2D). Collectively, these data suggest that the BM monocyte/macrophage percentage correlates with MPN phenotypes.
The detection and correlation analysis between the percentage of BM CD163+ monocytes/macrophages and MPN phenotype. A Representative plot of CD163 versus FSC-H of DAPI− BM monocytes/macrophages in HC, PV, ET, and PMF. B Quantitative analysis of CD163 proportion in HC (N = 6), PV (N = 10), ET (N = 10), and PMF (N = 8). C Representative cytospin images of BM monocytes/macrophages in HC, PV, ET, and PMF. D The correlation between RBC, HGB and the percentage of BM CD163+ monocytes/macrophages in PV patients (N = 10). E The correlation between PLT and the percentage of BM CD163+ monocytes/macrophages in ET patients (N = 10). F The correlation between WBC, RBC, HGB, PLT, and the percentage of BM CD163+ monocytes/macrophages in PMF patients (N = 8)
Significant transcriptional differences of monocytes/macrophages in PV patients compared to NBM
We then performed an RNA-seq analysis of BM monocytes/macrophages between treatment-naive PV patients and HCs to characterize the underlying molecular changes of the monocytes/macrophages. Principal component analysis (Fig. 2A) and hierarchical clustering analysis (Supplementary Fig. 3A) indicated that the monocytes/macrophages of treatment-naive PV patients clustered distinctly from those of HCs. A heatmap of the differential expression of genes between the two groups is shown in Fig. 2B. A total of 2,236 genes are differentially expressed, of which 1070 are upregulated and 1166 are downregulated in treatment-naive PV patients versus HCs (Supplementary Fig. 3B). All the differentially expressed genes are listed in Supplementary Table 1. Finally, the upregulated pathways of PV include cytokine–cytokine receptor interaction, cytokine and cytokine receptor, the Rap1 signaling pathway, the cGMP-PKG signaling pathway, platelet activation, and complement and coagulation cascades (Supplementary Fig. 3C). The downregulated pathways of PV include ribosome, cell cycle, oxidative phosphorylation, and the pathways of neurodegeneration-multiple diseases (Supplementary Fig. 3D).
Comparison of RNA-seq data of monocytes/macrophages between PV patients and healthy controls. A Principal component analysis between PV patients (N = 4) and healthy controls (N = 4). B Heatmap of the differentially expressed genes between PV patients (N = 4) and healthy controls (N = 4). C The FPKM value of cytokines and chemokines, CCL5, CXCL5, CXCL9, CXCL10, VEGF-C, and IL27 in PV and NBM. D The FPKM value of FCGR3A, CCR2, TLR1, TLR6, TLR8, and TLR9 in PV and NBM. E The FPKM value of C1QA, C1QB, and C2 in PV and NBM. F The FPKM value of APOL1, APOL2, APOL3, and APOL4 in PV and NBM. G The FPKM value of SLC25A16, SLC46A1, and SLC6A12 in PV and NBM. H The FPKM value of ITGA2, ITGA7, ITGB3, and ITGB5 in PV and NBM. I The FPKM value of NR1H3, CEBPA, and PPARGC1B in PV and NBM
Our research, as well as that of other groups, indicates that the expression levels of genes encoding proteins that are known to be important for the macrophage function of supporting erythropoiesis include adhesion molecules, molecules for nucleus engulfment and digestion, iron recycling molecules, and growth factors [18,19,20,21, 24, 29, 37,38,39,40,41,42]. Fig. 2C demonstrates the increased cytokines and chemokines, including CCL5, CXCL5, CXCL9, CXCL10, and VEGF-C. Monocytes/macrophages in treatment-naive PV patients also increased some of the receptors expressed by inflammatory monocytes/macrophages [5, 40, 43,44,45], including FCGR3A, CCR2, TLR1, TLR6, TLR7, TLR8, and TLR9 (Fig. 2D). C1QA, C1QB, and C2 are phagocytosis facilitators [46], which also increased in treatment-naive PV patients (Fig. 2E). APOL families play an important role in lipid metabolism and transport [47]. Significantly, the expression of APOL1, APOL2, APOL3, and APOL4 increased in treatment-naive PV patients (Fig. 2F). Solute carrier families play significant roles in ions exchange and homeostasis [48]. Interestingly, the expression of SLC25A16, SLC25A30, SLC46A1, and SLC6A12 also increased in treatment-naive PV patients (Fig. 2G). Integrins are known to participate in cell adhesion as well as cell-surface-mediated signaling [49]. We found that the expression of ITGA2, ITGA7, ITGB3, and ITGB5 increased significantly in treatment-naive PV patients (Fig. 2H). Gene expression is regulated by transcription factors. Finally, we demonstrated that the expression of the transcription factors NR1H3, CEBPA, and PPARGC1B increased significantly in treatment-naive PV patients (Fig. 2I).
Monocytes/macrophages promote ET via several distinct mechanisms compared to NBM
Using the same approach, we examined transcriptional alterations in monocytes/macrophages in the BM microenvironment of ET patients. Principal component analysis (Fig. 3A) and hierarchical clustering analysis (Supplementary Fig. 4A) indicated that treatment-naive ET patients cluster differently from HCs. A heatmap of the differential expression of genes is shown in Fig. 3B. A total of 2161 genes are differentially expressed, of which 1040 are upregulated and 1121 are downregulated in treatment-naive ET patients versus HCs (Supplementary Fig. 4B). All the differentially expressed genes are listed in Supplementary Table 2. The KEGG pathways analysis demonstrated that the upregulated pathways of ET include platelet activation, cell adhesion molecules, ECM-receptor interaction, focal adhesion, and the regulation of actin cytoskeleton (Supplementary Fig. 4C). The downregulated pathways of ET include viral protein interaction with cytokine and cytokine receptors, oxidative phosphorylation, thermogenesis, the NF-kappa B signaling pathway, the TNF signaling pathway, and the IL-17 signaling pathway (Supplementary Fig. 4D).
Comparison of RNA-seq data of monocytes/macrophages between ET patients and healthy controls. A Principal component analysis between ET patients (N = 3) and healthy controls (N = 4). B Heatmap of the differentially expressed genes between ET patients (N = 3) and healthy controls (N = 4). C The FPKM value of CCL5, PDGFA, PDGFB, VWF, FGF13, EGF, and IGF2 in ET and NBM. D The FPKM value of ITGA2, ITGA2B, ITGA6, and ITGA9 in ET and NBM. E The FPKM value of SLC40A1 in ET and NBM. F The FPKM value of MMP9 and MMP25 in ET and NBM. G The FPKM value of FOS, ARG1 and CEBPA in ET and NBM
Emerging evidence suggests that effective megakaryopoiesis depends on the homeostasis of the BM macrophage microenvironment [23, 50]. Fig. 3C demonstrates that the expression of CCL5, PDGFA, PDGFB, VWF, FGF13, EGF, and IGF2 increased significantly in treatment-naive ET patients. Integrins are known to mediate the adhesion of platelets and other cell types to the extracellular matrix to maintain the homeostasis of megakaryopoiesis [51]. Consistent with this, we found that the expression of ITGA2, ITGA2B, ITGA6, and ITGA9 increased significantly (Fig. 3D). SLC40A1 is involved in iron export from duodenal epithelial cells or macrophages, which is important for erythropoiesis [18]. Megakaryopoiesis also requires iron export macrophages. Significantly, the expression of SLC40A1 increased in treatment-naive ET patients (Fig. 3E). The matrix metalloproteinase (MMP) family is involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, tissue remodeling, and megakaryocytes migration [52,53,54]. Fig. 3F indicates that the expression of MMP9 and MMP25 increased significantly. Studies have indicated that M2 macrophages, but not M1 macrophages, are significant for megakaryopoiesis [23]. Consistent with this, the expression of the M2 macrophage marker ARG1 increased significantly (Fig. 3G). Two transcription factors, FOS and CEBPA expressions, were also found to be expressed in treatment-naive ET patients (Fig. 3G).
Monocytes/macrophages of PMF distinct heterogeneous expression of M1 and M2 markers compared to NBM
We examined the transcriptional alterations in monocytes/macrophages in the BM microenvironment of PMF patients. Principal component analysis (Fig. 4A) and hierarchical clustering analysis indicated that treatment-naïve clustering in PMF patients differ from clustering in HCs (Supplementary Fig. 5A). A heatmap of the differential expression of genes is shown in Fig. 4B. A total of 2252 genes are differentially expressed, of which 1347 are upregulated and 905 are downregulated in treatment-naive ET patients versus HCs (Supplementary Fig. 5B). All the differentially expressed genes are listed in Supplementary Table 3. The upregulated pathway of PMF includes cytokine–cytokine receptor interaction, platelet activation, a JAK-STAT signaling pathway, complement and coagulation cascades, and Fc gamma R-mediated phagocytosis (Supplementary Fig. 5C). However, the downregulated pathways only include the coronavirus disease COVID-19 and ribosome (Supplementary Fig. 5D).
Comparison of RNA-seq data of monocytes/macrophages between PMF patients and healthy controls. A Principal component analysis between PMF patients (N = 5) and healthy controls (N = 4). B Heatmap of the differentially expressed genes between PMF patients (N = 5) and healthy controls (N = 4). C The FPKM value of FCGR1A, FCGR1B, FCGR1C, FCGR3A, FCGR3B, HLA-DOA, HLA-DPA1, and HLA-DPB1 in PMF and NBM. D The FPKM value of STAT1, IFITM1, IFITM2, IFITM3, IFIT1, IFIT2, IFIT3, and IFIT27 in PMF and NBM. E The FPKM value of CCL5, GAS6, CCL25, CXCL9, CXCL10, CXCL12, VEGF-C, EGF, VWF, PDGFA, PDGFB, and IL27 in PMF and NBM. F The FPKM value of SIGLEC1, VCAM1, AXL, CD5L, and SLAMF7 in PMF and NBM. G The FPKM value of SPIC in PMF and NBM
It has been shown that macrophages increase in PMF [27]; however, transcriptional alterations in monocytes/macrophages in the BM microenvironment of PMF patients have not been performed. Figure 4C demonstrates that the expressions of FCGR1A, FCGR1B, FCGR1C, FCGR3A, FCGR3B, HLA-DOA, HLA-DPA1, and HLA-DPB1 increased significantly. Figure 4D indicates that many interferon-inducible genes, including STAT1, IFITM1, IFITM2, IFITM3, IFIT1, IFIT2, IFIT3, and IFIT27 increased significantly. We analyzed the production of cytokines and chemokines next and found that the expressions of CCL5, GAS6, CCL25, CXCL9, CXCL10, CXCL12, VEGF-C, EGF, VWF, PDGFA, PDGFB, and IL27 increased significantly (Fig. 4E). SIGLEC1, VCAM1, AXL, CD5L, and SLAMF7 were also found to be significantly increased (Fig. 4F), and, finally, we found that the transcription factor SPIC was also significantly increased (Fig. 4G).
Monocytes/macrophages in PMF, PV, and ET have relatively specific transcriptional profiles
We have shown that monocytes/macrophages in PMF, PV, and ET demonstrate distinct transcriptional expressed genes compared to NBM. To investigate the distinct transcriptional profiles of monocytes/macrophages in PMF, PV, and ET, we performed a two-by-two comparison of the transcriptional expression of monocytes/macrophages in the BM microenvironment of these three different types of MPN. Significantly, we still obtained similar results using this analysis (Supplementary 6A-F, Supplementary Tables 4, 5, and 6, Fig. 5A–H). Taken together, monocytes/macrophages may play relatively specific role in distinct MPNs.
Comparison of differential expression genes of monocytes/macrophages between PV, ET, and PMF patients. A The FPKM value of CCL18, CCL25, CXCL5, CXCL10, GAS6, IL15, IL27, IL18BP, and S100A12 in PV, ET and PMF. B The FPKM value of NR1H3, KLF1, HIF1α, MAFB, and ARG1 in PV, ET and PMF. C The FPKM value of STAT1, IRF1, IFITM3, IFI27, and AIM2 in PV, ET, and PMF. D The FPKM value of SLC2A5, SLC31A2, SLC40A1, and SLC6A12 in PV, ET and PMF. E The FPKM value of APOL1, APOL2, APOL3, and APOL4 in PV, ET and PMF. F The FPKM value of DNASE2, ICAM1, and MARCO in PV, ET and PMF. G The FPKM value of MMP8 and MMP9 in PV, ET, and PMF. H The FPKM value of HLA-DPB1, HLA-DQA1, HLA-DRB1, HLA-DMB, HLA-DRA, HLA-A, HLA-DMA and CD74 in PV, ET, and PMF
Discussion
The classical view is that malignant clonal expansion of hematopoietic stem/progenitor cells is the central driver of MPN development, but the same JAK2V617F mutation allows MPN to manifest in three clinical phenotypes, namely PV, ET, and PMF [9,10,11,12]. Studies have illustrated the complexity of MPN pathogenesis and the heterogeneity of clinical manifestations well, and there is increasing evidence that the BM microenvironments may play important roles [55,56,57]. Medical interventions, immune dysregulation, and inflammation have all been shown to alter selective pressures for mutant clones in the BM. Transcriptome analysis of PBMCs has shown that MPN patients are significantly enriched for genes related to inflammation, immune response, and oxidative stress [58]. Immune infiltration analysis as well as mathematical modeling studies have also suggested the presence of complex immune remodeling in the MPN microenvironment [59, 60]. It has also been shown that there is significant immune activation in the microenvironments of MPN patients, including decreased levels of anti-inflammatory cytokines, increased levels of pro-inflammatory cytokines, as well as a significant Th1/Th2 immune bias [61]. Despite these studies, however, the role of monocytes/macrophages in the BM microenvironment in MPN is still unclear. In the present study, we found that CD163+ monocytes/macrophages in the BM of MPN increased significantly compared to HCs. Interestingly, there was significant variability in the morphology of monocytes/macrophages in different subtypes of MPN, suggesting that monocytes/macrophages may have relatively specific functions in the different subtypes of MPN. Correlation analyses showed a positive correlation between monocyte/macrophage counts and elevated hemoglobin in patients with PV. Monocyte/macrophage counts in ET patients were positively correlated with elevated platelets. In contrast, the monocyte/macrophage count in PMF patients was positively correlated with a decrease in hemoglobin. Overall, our study suggests that the BM monocyte/macrophage may associate to the development of MPN.
CD14+CD16+ monocytes/macrophages are thought to be involved in a variety of inflammation-related diseases in humans [62,63,64,65]. Bassan et al. reported that the peripheral blood CD14+CD16+ monocytes were significantly elevated in different MPN subtypes [66]. Importantly, the JAK2V617F mutation was significantly associated with the percentage of CD14+CD16+ monocytes. Borges et al. demonstrated that the peripheral blood CD14+CD16+ non-classical monocytes significantly expressed CD169, CD206, CD163, SIRPα, and CD106, which are involved in erythropoiesis, in PV [25]. Despite these findings, studies have mainly focused on peripheral blood inflammatory monocytes in MPN. In contrast, alterations in the immune microenvironment of the BM may be more influential in the development and progression of MPN. However, the role of CD14+CD16+ inflammatory monocytes/macrophages in the BM microenvironment in MPN is still unclear. In this study, we found that the percentage of CD14+CD16+ inflammatory monocytes/macrophages in BM increases in the three subtypes of MPN. Interestingly, the percentage of BM CD14+CD16+ inflammatory monocytes/macrophages was positively associated with hemoglobin in PV and platelets in ET. In contrast, the percentage of BM CD14+CD16+ inflammatory monocytes/macrophages was negatively associated with hemoglobin in PMF. In conclusion, our study demonstrates for the first time that inflammatory monocytes/macrophages are elevated in different subtypes of MPN and further confirms that the BM microenvironment of MPN presents an inflammatory microenvironmental state.
Macrophages can promote erythropoiesis through a number of different mechanisms [18,19,20, 24, 29, 39, 40]. The removal of macrophages from PV mice resulted in a significant decrease in erythrocytes and hemoglobin, suggesting that macrophages are also involved in the development of PV [67]. In the present study, we performed RNA-seq of CD163+ monocytes/macrophages from PV and NBM. Our study found that several cytokines and chemokines, such as CCL5, CXCL5, CXCL9, CXCL10, VEGF-C and IL27, increased significantly compared to NBM. Fang et al. demonstrated that VEGF-C is essential for the mobilization, maturation, and enucleation of primitive erythroblasts, and VEGF-C deletion compromises liver colonization by erythro-myeloid progenitors and subsequent macrophage/erythroid expansion [68]. The same group also indicates that VEGF-C deletion in endothelial or LepR+ cells compromises the bone marrow perivascular niche and hematopoietic stem cell maintenance and exogenously administered VEGF-C improves hematopoietic recovery after irradiation by accelerating endothelial and LepR+ cell regeneration [69]. Therefore, VEGF-C is significant for erythropoiesis, and our study extends the understanding of the role of monocytes/macrophages’ VEGF-C in the development of PV. Our study indicates that GM-CSF causes erythrocytopenia by affecting the formation of EBI [29]. IL27 suppresses the GM-CSF expression [70]. Therefore, IL27 may be a novel cytokine that can promote the production of red blood cells, and further studies should be performed to confirm this. Furthermore, it would also be worth examining the roles of other cytokines or chemokines that are enhanced in the development of PV. The immune microenvironment of PV patients is an inflammatory microenvironment, and an inflammatory microenvironment in BM inhibits erythropoiesis [29, 71] and induces extramedullary erythropoiesis [40, 72]. This is also illustrated by our data on the elevated expression of TLRs and FCGR3A in PV. CCR2 is significant for the homing of HSCs/HPCs to sites of inflammation [44], and CCR2+ monocyte-derived macrophages expand the murine stress erythropoietic niche during recovery from anemia [40]. In conjunction with our finding of an elevated monocyte/macrophage CCR2 expression in the BM microenvironment of PV patients, we hypothesize that PV patients may also have an inflammatory microenvironment that recruits CCR2+ monocytes into the BM microenvironment to differentiate into EBI macrophages that promote erythropoiesis. This should be verified in the future in a mouse model of PV. C1QA [73], C1QB [74], and C2 [74] are significant for phagocytosis. APOL1, APOL2, APOL3, and APOL4 are significant for lipid metabolism [47, 75]. SLC25A16, SLC46A1, and SLC6A12 are significant for ion transport, and ITGA2, ITGA7, ITGB3, and ITGB5 are significant for cell adhesion. These genes may be involved in the occurrence and progression of PV; however, more research should be conducted to demonstrate this. Furthermore, the increased expression of transcription factors in PV included Nr1H3, CEBPA, and PPARGC1B. The exact role of these genes in PV is unclear, and more studies should be conducted to confirm the role of these genes in PV.
Huang’s group report that TGF-β released by M2 macrophages may facilitate megakaryopoiesis through the upregulation of the JAK2/STAT5 and MAPK/ERK pathways in megakaryocytes [23]. In the present study, the expression of ARG1, a specific marker for M2 macrophages, was significantly enhanced in ET. The finding in our study confirms that macrophages in ET patients incline more to the M2 type. We also found that several cytokines and chemokines expressed by monocytes/macrophages were significantly enhanced in ET patients. Previous studies have indicated that CCL5 enhances megakaryocyte differentiation and development [76,77,78,79]. We found in our study that CCL5 expression by monocytes/macrophages was significantly elevated in ET, which further demonstrates that monocytes/macrophages can promote platelet production by secreting CCL5. Previous studies have also found that EGF transcript levels and cytokine levels from platelets were significantly elevated in ET [80, 81]. Our study extends the origin of EGF to monocytes/macrophages in ET. MMP9 is involved in platelet formation and the migration of megakaryocytes [52, 53, 82]. We found that monocyte/macrophage expression of MMP9 was significantly elevated in ET patients, which revealed a novel mechanism of platelet production promotion by monocytes/macrophages. Other cytokines, such as PDGFA, PDGFB, VWF, FGF13, and IGF2; cell adhesion molecules, such as ITGA2, ITGA2B, ITGA6, and ITGA9; the iron exporter gene, SLC40A1, matrix metalloproteinase family gene; MMP25 and transcription factors FOS, and CEBPA have also been found to be increased in ET patients. However, the exact roles of these genes expressed by monocytes/macrophages in ET are unclear, and more studies should be conducted to confirm the role of these genes in ET.
Several studies on mouse models have been used to explain the mechanism of myelofibrosis development caused by megakaryocytes [83, 84], stromal cells [85, 86], monocytes [4, 87], and macrophages [26, 88]. However, the transcriptional expression of monocytes/macrophages in PMF patients is still not completely clear. In the present study, we found that the expression of FCGR1A, FCGR1B, FCGR1C, FCGR3A, FCGR3B, HLA-DOA, HLA-DPA1, and HLA-DPB1; the interferon-inducible genes STAT1, IFITM1, IFITM2, IFITM3, IFIT1, IFIT2, and IFIT3; and IFI27 by monocytes/macrophages increased significantly in PMF. These genes expressed by monocytes/macrophages correlate with an inflammatory microenvironment. Our study further confirms that PMF bone marrow microenvironment is an inflammatory microenvironment. Studies have indicated that PDGF-A and -B, as well as PDGF receptor α (PDGFRα) and PDGF receptor β (PDGFRβ) expression is increased in the BM of PMF patients [89]. Decker et al. identified Lepr+ stromal lineage cells as the origin of myofibroblasts in PMF. The PDGFRA pathway in BM Lepr+ cells lead to the expansion of these cells and extramedullary hematopoiesis, and this suggests that targeting PDGFRA signaling could be an effective way to treat BM fibrosis [86]. Kramer et al. have demonstrated that PDGFRβ and PDGF-B protein expression in overt fibrotic BM and PDGFRβ–PDGF-B interaction were significantly increased [90]. In the present study, we found that the expression of PDGFA and PDGFB derived from monocytes/macrophages increased significantly. Mouse models should also confirm the roles of PDGFA and PDGFB derived from monocytes/macrophages in PMF. The expression of CXCL12 by perivascular mesenchymal stromal cells (MSCs) plays an essential role in HSC maintenance [91,92,93]. Wang et al. report that the splenic microenvironment in MF is characterized by increased levels of intact, functional CXCL12, which contributes to the localization of MF CD34+ cells to the spleen and the establishment of extramedullary hematopoiesis [94]. In the present study, we found that CXCL12 expression by monocytes/macrophages significantly increase in PMF patients. Therefore, we hypothesize that elevated CXCL12 expression may play a compensatory hematopoietic role in the PMF BM hematopoietic microenvironment, and further studies should be conducted to confirm the role of CXCL12 expressed by BM monocytes/macrophages in PMF mouse models. The expression of CCL5 and CXCL9 were also found to be increased in PMF mouse models [95]. Our study confirms that the expression of CCL5 and CXCL9 from monocytes/macrophages also increased in PMF patients. Beitzen-Heineke et al. found that AXL is abundantly activated in MPN cells and that its ligand growth arrest-specific gene 6 is upregulated in MPN patients [96]. The findings from our present study, which involved the expression of AXL and GAS6 from monocytes/macrophages in PMF patients, are consistent with their findings. Xu et al. report that the soluble vascular cell adhesion molecule-1 (sVCAM-1) increases dramatically in myelofibrosis [97]. Increased VCAM1 expression may contribute to the retention of hematopoietic stem cells/PCs in the spleen and play a role in the initiation of extramedullary hematopoiesis in the early stages of MF [98, 99]. Our study found that VCAM1 expressed by monocytes/macrophages in PMF increases significantly. Further studies should be conducted to examine the roles of monocytes/macrophages VCAM1 in PMF models. Maekawa et al. found that SLAMF7high monocytes increase in the PB of patients with MF, in correlation with the JAK2V617F mutation and anti-SLAMF7 antibodies suppressed monocyte-derived fibrocyte differentiation and could be a potent therapeutic agent for MF [4]. In this study, we found that the expression of SLAMF7 in monocytes/macrophages increase significantly in the BM of PMF patients with the JAK2V617F mutation and confirmed that SLAMF7 could be a new therapeutic target for PMF.
In normal conditions, the expression of SPIC by macrophages is significant for iron metabolism [100, 101]. Our previous study also demonstrates that EBI macrophages, but not non-EBI macrophages, express SPIC, which provides iron for erythropoiesis [18]. Inflammatory disorders and infections are associated with cytopenias, including anemia and thrombocytopenia [43]. In a mouse model of macrophage activation syndrome, Akilesh et al. demonstrated that TLR7 or TLR9 signaling in monocytes causes these cells to differentiate into inflammatory hematophagocytes (iHPCs), which highly expressed SPIC [43], and the iHPCs showed higher phagocytic uptake of RBCs [43]. In combination, the increased expression of SPIC in PMF may share similar function to inflammatory disorders. Further studies are also required to examine the exact role of SPIC in PMF models.
Studies have shown that PV is more easily converted into PMF than ET and that the BM microenvironment may play an important role in this process [102, 103]. We compared the transcriptional differences between the monocytes/macrophages in PV, ET, and PMF. Consistent with the easier conversion of PV to PMF, the transcriptional profile of monocytes/macrophages in PV is more similar to those of PMF relative to ET. The differences in the monocyte/macrophage expression profiles in PMF compared to ET patients were both different and identical to those in PMF and in healthy controls. These genes included CCL25, CXCL10, GAS6, IL27, NR1H3, MAFB, STAT1, IFITM3, IFI27, SLC31A2, SLC6A12, APOL1, APOL2, APOL3, APOL4, ICAM1, MARCO, HLA-DPA1, HLA-DPB1, HLA-DQA1, HLA-DRB1, HLA-DMB, HLA-DRA, HLA-A, HLA-DMA, and CD74. According to our study, it seems that many genes of monocytes/macrophages could be involved in PV, ET, and PMF and play similar or specific roles in these three MPN subtypes. Further studies should be performed to confirm the roles of these genes in distinct types of MPN.
However, this study has limitations. The way that differentially expressed genes regulate the development of distinct MPNs should be studied further. Single-cell sequencing of monocytes/macrophages in different subtypes of MPN should also be performed to investigate the similarities and differences in monocytes/macrophages in different subtypes of MPN in-depth. And independent studies are required to confirm the findings of this study. Nevertheless, we have characterized that BM CD163 or CD14 in combination with CD16 expression in monocytes/macrophages increase and may correlate with the clinical phenotypes of MPN. Transcriptome sequencing of monocytes/macrophages in PV, ET, and PMF also showed relatively specific transcriptional profiles of monocytes/macrophages in these three types of MPN, which suggests that monocyte/macrophage-mediated immune remodeling of different subtypes of MPN may also plays a role in MPN.
Availability of data and materials
All data generated and materials in the study are included in the present article and supplementary data.
Abbreviations
- ET :
-
Essential thrombocytosis
- HCs :
-
Healthy controls
- HSC :
-
Hematopoietic stem cells
- iHPCs :
-
Inflammatory hematophagocytes
- MSCs :
-
mesenchymal stromal cells
- Mk :
-
megakaryocyte
- MPNs :
-
myeloproliferative neoplasms
- NBM :
-
normal bone marrow
- PV :
-
polycythemia vera
- PMF :
-
primary myelofibrosis
- sVCAM-1 :
-
soluble vascular cell adhesion molecule-1
References
Tefferi A (2016) Myeloproliferative neoplasms: a decade of discoveries and treatment advances. Am J Hematol 91(1):50–58
Dameshek W (1951) Some speculations on the myeloproliferative syndromes. Blood 6(4):372–375
Tong J, Sun T, Ma S et al (2021) Hematopoietic stem cell heterogeneity is linked to the initiation and therapeutic response of myeloproliferative neoplasms. Cell Stem Cell 28(3):502-513.e6
Maekawa T, Kato S, Kawamura T et al (2019) Increased SLAMF7high monocytes in myelofibrosis patients harboring JAK2V617F provide a therapeutic target of elotuzumab. Blood 134(10):814–825
Lai HY, Brooks SA, Craver BM et al (2019) Defective negative regulation of Toll-like receptor signaling leads to excessive TNF-α in myeloproliferative neoplasm. Blood Adv 3(2):122–131
Guy A, Poisson J, James C (2021) Pathogenesis of cardiovascular events in BCR-ABL1-negative myeloproliferative neoplasms. Leukemia 35(4):935–955
Skoda RC, Duek A, Grisouard J (2015) Pathogenesis of myeloproliferative neoplasms. Exp Hematol 43(8):599–608
Pikman Y, Lee BH, Mercher T et al (2006) MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 3(7):e270
Baxter EJ, Scott LM, Campbell PJ et al (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet (London, England) 365(9464):1054–1061
Kralovics R, Passamonti F, Buser AS et al (2005) A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 352(17):1779–1790
Levine RL, Wadleigh M, Cools J et al (2005) Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7(4):387–397
James C, Ugo V, Le Couédic JP et al (2005) A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434(7037):1144–1148
Luque Paz D, Ashcroft P, Skoda RC (2021) Myeloproliferative neoplasms: the long wait for JAK2-mutant clone expansion. Cell Stem Cell 28(3):359–361
Morrison SJ, Scadden DT (2014) The bone marrow niche for haematopoietic stem cells. Nature 505(7483):327–334
Kunisaki Y, Frenette PS (2012) The secrets of the bone marrow niche: enigmatic niche brings challenge for HSC expansion. Nat Med 18(6):864–865
Méndez-Ferrer S, Michurina TV, Ferraro F et al (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466(7308):829–834
Li W, Wang F, Guo R, Bian Z, Song Y (2022) Targeting macrophages in hematological malignancies: recent advances and future directions. J Hematol Oncol 15(1):110
Li W, Wang Y, Zhao H et al (2019) Identification and transcriptome analysis of erythroblastic island macrophages. Blood 134(5):480–491
Li W, Wang Y, Chen L, An X (2019) Erythroblast island macrophages: recent discovery and future perspectives. Blood Sci 1(1):61–64
Li W, Guo R, Song Y, Jiang Z (2021) Erythroblastic island macrophages shape normal erythropoiesis and drive associated disorders in erythroid hematopoietic diseases. Front Cell Dev Biol 8:1858
Chow A, Lucas D, Hidalgo A et al (2011) Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 208(2):261–271
Naik S, Larsen SB, Cowley CJ, Fuchs E (2018) Two to tango: dialog between immunity and stem cells in health and disease. Cell 175(4):908–920
Zhao H-Y, Zhang Y-Y, Xing T et al (2021) M2 macrophages, but not M1 macrophages, support megakaryopoiesis by upregulating PI3K-AKT pathway activity. Signal Transduct Target Ther 6(1):234
Chen Y, Xiang J, Qian F et al (2020) Epo receptor signaling in macrophages alters the splenic niche to promote erythroid differentiation. Blood 136(2):235–246
Borges MD, Sesti-Costa R, de Albuquerque DM et al (2021) Monocytes from patients with polycythemia vera express molecules related to stress erythropoiesis and have increased erythrocyte phagocytosis. Blood 138:1466
Wakahashi K, Minagawa K, Kawano Y et al (2019) Vitamin D receptor-mediated skewed differentiation of macrophages initiates myelofibrosis and subsequent osteosclerosis. Blood 133(15):1619–1629
Molitor DCA, Boor P, Buness A et al (2021) Macrophage frequency in the bone marrow correlates with morphologic subtype of myeloproliferative neoplasm. Ann Hematol 100(1):97–104
Van Egeren D, Kamaz B, Liu S et al (2021) Transcriptional differences between JAK2-V617F and wild-type bone marrow cells in patients with myeloproliferative neoplasms. Exp Hematol. 107:14–19
Cao W, Fan W, Wang F et al (2022) GM-CSF impairs erythropoiesis by disrupting erythroblastic island formation via macrophages. J Transl Med 20(1):11
Xu L, Yan M, Long J et al (2022) Identification of macrophage correlated biomarkers to predict the prognosis in patients with intrahepatic cholangiocarcinoma. Front Oncol 12:967982
Wang Y, Li W, Schulz VP et al (2021) Impairment of human terminal erythroid differentiation by histone deacetylase 5 deficiency. Blood 138(17):1615–1627
Xu L, Yan M, Li W (2022) Matrix metalloproteinase 1 is a poor prognostic biomarker for patients with hepatocellular carcinoma (Clin Exp Med.2022 Sep 28)
Yang H, Yan M, Li W, Xu L (2022) SIRPα and PD1 expression on tumor-associated macrophage predict prognosis of intrahepatic cholangiocarcinoma. J Transl Med 20(1):140
Li W, Li T, Sun C et al (2022) Identification and prognostic analysis of biomarkers to predict the progression of pancreatic cancer patients. Mol Med 28(1):43
Schinke C, Poos AM, Bauer M et al (2022) Characterizing the role of the immune microenvironment in multiple myeloma progression at a single-cell level. Blood Adv 6(22):5873–5883
Shi H, He H, Cui L et al (2021) Transcriptomic landscape of circulating mononuclear phagocytes in Langerhans cell histiocytosis at the single-cell level. Blood 138(14):1237–1248
Chasis JA, Mohandas N (2008) Erythroblastic islands: niches for erythropoiesis. Blood 112(3):470–478
Fabriek BO, Polfliet MMJ, Vloet RPM et al (2007) The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor. Blood 109(12):5223–5229
Wei Q, Boulais PE, Zhang D et al (2019) Maea expressed by macrophages, but not erythroblasts, maintains postnatal murine bone marrow erythroblastic islands. Blood 133(11):1222–1232
Liao C, SandeepPrabhu K, Paulson RF (2018) Monocyte-derived macrophages expand the murine stress erythropoietic niche during the recovery from anemia. Blood 132(24):2580–2593
Kusakabe M, Hasegawa K, Hamada M et al (2011) c-Maf plays a crucial role for the definitive erythropoiesis that accompanies erythroblastic island formation in the fetal liver. Blood 118(5):1374–1385
Chow A, Huggins M, Ahmed J et al (2013) CD169 + macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med 19(4):429–436
Akilesh H M, Buechler M B, Duggan J M (2019) Chronic TLR7 and TLR9 signaling drives anemia via differentiation of specialized hemophagocytes. Science 363(6423):eaao5213
Si Y, Tsou C-L, Croft K, Charo IF (2010) CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest 120(4):1192–1203
Ziegler-Heitbrock L (2007) The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 81(3):584–592
Galvan MD, Greenlee-Wacker MC, Bohlson SS (2012) C1q and phagocytosis: the perfect complement to a good meal. J Leukoc Biol 92(3):489–497
Duchateau PN, Pullinger CR, Cho MH, Eng C, Kane JP (2001) Apolipoprotein L gene family: tissue-specific expression, splicing, promoter regions; discovery of a new gene. J Lipid Res 42(4):620–630
Wu Z, Xu J, Liang C et al (2021) Emerging roles of the solute carrier family in pancreatic cancer. Clin Transl Med 11(3):e356
Laskowska J, Szczepanek J, Tretyn A, Styczynski J (2015) Identification of the genomic rearrangements associated with the ex vivo resistance to anthracyclines in childhood acute leukemias. Blood 126(23):4997
Hou M (2021) Regulation of megakaryopoiesis by bone marrow macrophage polarization. Blood Sci 03(04):149–150
Yang X, Chitalia SV, Matsuura S, Ravid K (2022) Integrins and their role in megakaryocyte development and function. Exp Hematol 106:31–39
Lane WJ, Dias S, Hattori K et al (2000) Stromal-derived factor 1–induced megakaryocyte migration and platelet production is dependent on matrix metalloproteinases. Blood 96(13):4152–4159
Cecchetti L, Tolley ND, Michetti N et al (2011) Megakaryocytes differentially sort mRNAs for matrix metalloproteinases and their inhibitors into platelets: a mechanism for regulating synthetic events. Blood 118(7):1903–1911
Page-McCaw A, Serano J, Santé JM, Rubin GM (2003) Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev Cell 4(1):95–106
Schepers K, Pietras EM, Reynaud D et al (2013) Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 13(3):285–299
Koschmieder S, Chatain N (2020) Role of inflammation in the biology of myeloproliferative neoplasms. Blood Rev 42:100711
Curto-Garcia N, Harrison C, McLornan DP (2020) Bone marrow niche dysregulation in myeloproliferative neoplasms. Haematologica 105(5):1189–1200
Hasselbalch HC, Thomassen M, Riley CH et al (2014) Whole blood transcriptional profiling reveals deregulation of oxidative and antioxidative defence genes in myelofibrosis and related neoplasms. Potential implications of downregulation of Nrf2 for genomic instability and disease progression. PLoS One 9(11):e112786
Andersen M, Sajid Z, Pedersen RK et al (2017) Mathematical modelling as a proof of concept for MPNs as a human inflammation model for cancer development. PLoS One 12(8):e0183620
Mendez Luque LF, Blackmon AL, Ramanathan G, Fleischman AG (2019) Key role of inflammation in myeloproliferative neoplasms: instigator of disease initiation, progression and symptoms. Curr Hematol Malig Rep 14(3):145–153
Sun T, Ju M, Dai X et al (2020) Multilevel defects in the hematopoietic niche in essential thrombocythemia. Haematologica 105(3):661–673
Devisscher L, Verhelst X, Colle I, Van Vlierberghe H, Geerts A (2016) The role of macrophages in obesity-driven chronic liver disease. J Leukoc Biol 99(5):693–698
Hénaut L, Candellier A, Boudot C et al (2019) New insights into the roles of monocytes/macrophages in cardiovascular calcification associated with chronic kidney disease. Toxins (Basel). 11(9):529
Zhao W, Beers DR, Hooten KG et al (2017) Characterization of gene expression phenotype in amyotrophic lateral sclerosis monocytes. JAMA Neurol 74(6):677–685
Tu J, Huang W, Zhang W, Mei J, Zhu C (2021) A Tale of two immune cells in rheumatoid arthritis: the crosstalk between macrophages and T cells in the synovium. Front Immunol 12:655477
Bassan VL, Barretto GD, de Almeida FC et al (2022) Philadelphia-negative myeloproliferative neoplasms display alterations in monocyte subpopulations frequency and immunophenotype. Med Oncol 39(12):223
Ramos P, Casu C, Gardenghi S et al (2013) Macrophages support pathological erythropoiesis in polycythemia vera and β-thalassemia. Nat Med 19(4):437–445
Fang S, Nurmi H, Heinolainen K et al (2016) Critical requirement of VEGF-C in transition to fetal erythropoiesis. Blood 128(5):710–720
Fang S, Chen S, Nurmi H et al (2020) VEGF-C protects the integrity of the bone marrow perivascular niche in mice. Blood 136(16):1871–1883
Young A, Linehan E, Hams E et al (2012) Cutting edge: suppression of GM-CSF expression in murine and human T cells by IL-27. J Immunol 189(5):2079–2083
Mei Y, Zhao B, Basiorka AA et al (2018) Age-related inflammatory bone marrow microenvironment induces ineffective erythropoiesis mimicking del(5q) MDS. Leukemia 32(4):1023–1033
Müller P, Baldauf CK, Haage TR et al (2021) Anti-inflammatory treatment in MPN: targeting TNFR1 and TNFR2 in JAK2-V617F–induced disease. Blood Adv 5(23):5349–5359
Hodrea J, Majai G, Doró Z et al (2012) The glucocorticoid dexamethasone programs human dendritic cells for enhanced phagocytosis of apoptotic neutrophils and inflammatory response. J Leukoc Biol 91(1):127–136
Mukundan L, Odegaard JI, Morel CR et al (2009) PPAR-δ senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med 15(11):1266–1272
Monajemi H, Fontijn RD, Pannekoek H, Horrevoets AJG (2002) The apolipoprotein L gene cluster has emerged recently in evolution and is expressed in human vascular tissue. Genomics 79(4):539–546
Machlus KR, Johnson KE, Kulenthirarajan R et al (2015) CCL5 Released from platelets increases megakaryocyte maturation and proplatelet formation. Blood 126(23):1162
Machlus KR, Johnson KE, Kulenthirarajan R et al (2016) CCL5 derived from platelets increases megakaryocyte proplatelet formation. Blood 127(7):921–926
Noetzli L, Parvin S, Battinelli E, Machlus K (2018) CCL5 Enhances megakaryocyte differentiation and development. Blood 132:522
Stone AP, Nascimento TF, Barrachina MN (2022) The bone marrow niche from the inside out: how megakaryocytes are shaped by and shape hematopoiesis. Blood 139(4):483–491
Collinson RJ, Mazza-Parton A, Fuller KA et al (2020) Gene expression of CXCL1 (GRO-α) and EGF by platelets in myeloproliferative neoplasms. HemaSphere 4(6):e490
Øbro NF, Grinfeld J, Belmonte M et al (2020) Longitudinal cytokine profiling identifies GRO-α and EGF as potential biomarkers of disease progression in essential thrombocythemia. HemaSphere 4(3):e371
Mannello F, Medda V (2011) Differential expression of MMP-2 and MMP-9 activity in megakaryocytes and platelets. Blood 118(24):6470–6471
Varricchio L, Iancu-Rubin C, Upadhyaya B et al (2021) TGF-β1 protein trap AVID200 beneficially affects hematopoiesis and bone marrow fibrosis in myelofibrosis. JCI insight. 6(18)
Arciprete F, Zingariello M, Mazzarini M et al (2022) The immature morphology of the megakaryocytes present in the bone marrow of patients with myelofibrosis reflects changes in the frequency of functionally distinctive subpopulations. Blood 140(Supplement 1):12148–12149
Martinaud C, Desterke C, Konopacki J et al (2015) Osteogenic potential of mesenchymal stromal cells contributes to primary myelofibrosis. Cancer Res 75(22):4753–4765
Decker M, Martinez-Morentin L, Wang G et al (2017) Leptin-receptor-expressing bone marrow stromal cells are myofibroblasts in primary myelofibrosis. Nat Cell Biol 19(6):677–688
Barone M, Catani L, Ricci F et al (2020) The role of circulating monocytes and JAK inhibition in the infectious-driven inflammatory response of myelofibrosis. Oncoimmunology 9(1):1782575
Shinoda D, Nakajima-Takagi Y, Oshima M et al (2022) Insufficiency of non-canonical PRC1 synergizes with JAK2V617F in the development of myelofibrosis. Leukemia 36(2):452–463
Lev PR, Salim JP, Kornblihtt LI et al (2005) PDGF-A, PDGF-B, TGFβ, and bFGF mRNA levels in patients with essential thrombocythemia treated with anagrelide. Am J Hematol 78(2):155–157
Kramer F, Dernedde J, Mezheyeuski A et al (2020) Platelet-derived growth factor receptor β activation and regulation in murine myelofibrosis. Haematologica 105(8):2083–2094
Ding L, Saunders TL, Enikolopov G, Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481(7382):457–462
Greenbaum A, Hsu Y-MS, Day RB et al (2013) CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495(7440):227–230
Ding L, Morrison SJ (2013) Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495(7440):231–235
Wang X, Cho SY, Hu CS et al (2015) C-X-C motif chemokine 12 influences the development of extramedullary hematopoiesis in the spleens of myelofibrosis patients. Exp Hematol 43(2):100-109.e1
Kleppe M, Kwak M, Koppikar P et al (2015) JAK–STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov 5(3):316–331
Beitzen-Heineke A, Berenbrok N, Waizenegger J et al (2021) AXL inhibition represents a novel therapeutic approach in BCR-ABL negative myeloproliferative neoplasms. HemaSphere 5(9):e630
Xu M, Bruno E, Chao J et al (2005) Constitutive mobilization of CD34+ cells into the peripheral blood in idiopathic myelofibrosis may be due to the action of a number of proteases. Blood 105(11):4508–4515
Dutta P, Hoyer FF, Grigoryeva LS et al (2015) Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. J Exp Med 212(4):497–512
Qiu J, Salama ME, Hu CS et al (2018) The characteristics of vessel lining cells in normal spleens and their role in the pathobiology of myelofibrosis. Blood Adv 2(10):1130–1145
Kohyama M, Ise W, Edelson BT et al (2009) Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature 457(7227):318–321
Haldar M, Kohyama M, So AYL et al (2014) Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell 156(6):1223–1234
Tefferi A, Guglielmelli P, Larson DR et al (2014) Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 124(16):2507–13; quiz 2615
Silver RT (2014) Life, genes, and death in Ph- MPNs. Blood 124(16):2471–2472
Acknowledgements
We thank all our authors listed in this manuscript.
Funding
This work was supported by the Natural Science Foundation of China (82270149, 82270141, 32100698, 82170211), China Postdoctoral Science Foundation (2022T150592, 2021M692930) and Young Postdoctoral Innovators in Henan Province (WL). Key research and development and promotion of special projects in Henan Province (222102310204). Henan Province Medical Science and Technology Research Project (SBGJ202102146, LHGJ20220304, LHGJ20220305). Natural Science Foundation of Henan Province (222300420567). Outstanding Youth Fund of Henan Province (222300420068). Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University.
Author information
Authors and Affiliations
Contributions
WF, WC, and FG designed experiments, performed experiments, and analyzed the data. JS analyzed the RNA-seq data. MW, LX, FW, YL, RG, and ZB designed and supervised the study and edited the manuscript. WL drafted manuscript and edited the manuscript. ZJ and WM revised and edited the manuscript. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was reviewed and approved by The First Affiliated Hospital of Zhengzhou University in Zhengzhou, Henan Province, China (2021-KY-0575–002).
Consent for publication
Informed consent was obtained from all patients for being included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenjuan Fan, Weijie Cao, Jianxiang Shi, and Fengcai Gao contribute the same to this paper.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary Fig. 1
The clinical phenotype of MPNs. (A) Blood routine test of MPNs (85 of PV patients, 131 of ET patients and 42 of PMF patients), including RBC, HGB, HCT, Reti, PLT and WBC. (B) The representative HE staining of post-bone marrow biopsies of different types of MPN. (PNG 785 kb)

Supplementary Fig. 2
The detection of CD14+CD16+subset in CD163+ monocytes/macrophages in MPN and correlation analyses between the proportion of BM CD14+CD16+ monocytes/macrophages and MPN phenotype. (A) Representative plot of CD14 versusCD16 of DAPI-CD163+BM monocytes/macrophages in HC, PV, ET and PMF. (B) Quantitative analysis of CD14+CD16+ proportion in HC(N=6), PV(N=10), ET(N=10) and PMF(N=8). (C) The correlation between RBC, HGB and the percentage of BM CD14+CD16+ monocytes/macrophages in PV patients(N=10). (D) The correlation between PLT and the percentage of BM CD14+CD16+ monocytes/macrophages in ET patients(N=10). (E) The correlation between BC, RBC, HGB, PLT and the percentage of BM CD14+CD16+ monocytes/macrophages in PMF patients(N=8). (PNG 216 kb)

Supplementary Fig. 3
Comparison of differential expression genes and KEGG pathways of BM monocytes/macrophages between PV patients and healthy controls. (A) Hierarchical clustering analysis between PV patients (N=4) and healthy controls (N=4).(B) Numbers of unregulated and downregulated genes between PV patients (N=4) and healthy controls (N=4). (C) The upregulated KEGG pathway in PV patients compared to NBM. (D) The downregulated KEGG pathway in PV patients compared to NBM. (PNG 217 kb)

Supplementary Fig. 4
Comparison of differential expression genes and KEGG pathways of BM monocytes/macrophages between ET patients and healthy controls. (A) Hierarchical clustering analysis between ET patients (N=3) and healthy controls (N=4).(B) Numbers of unregulated and downregulated genes between ET patients (N=3) and healthy controls (N=4). (C) The upregulated KEGG pathway in ET patients compared to NBM. (D) The downregulated KEGG pathway in ET patients compared to NBM. (PNG 202 kb)

Supplementary Fig. 5
Comparison of differential expression genes and KEGG pathways of BM monocytes/macrophages between PMF patients and healthy controls. (A) Hierarchical clustering analysis between PMF patients (N=5) and healthy controls (N=4).(B) Numbers of unregulated and downregulated genes between PMF patients (N=5) and healthy controls (N=4).(C) The upregulated KEGG pathway in PMF patients compared to NBM. (D) The downregulated KEGG pathway in PMF patients compared to NBM. (PNG 228 kb)

Supplementary Fig. 6
Comparison of differential expression genes of monocytes/macrophages between PV, ET and PMF patients. (A) Principal component analysis between PMF patients (N=5) and PV patients (N=4). (B) Heatmap of the differentially expressed genes between PMF patients (N=5) and PV patients (N=4). (C) Principal component analysis between PMF patients (N=5) and ET patients (N=3). (D) Heatmap of the differentially expressed genes between PMF patients (N=5) and ET patients (N=3). (E) Principal component analysis between PV patients (N=4) and ET patients (N=3). (F) Heatmap of the differentially expressed genes between PV patients (N=4) and ET patients (N=3). (PNG 147 kb)
Supplementary Table 1
The upregulated and downregulated genes in PV compared to NBM. (CSV 686 kb)
Supplementary Table 2
The upregulated and downregulated genes in ET compared to NBM. (CSV 627 kb)
Supplementary Table 3
The upregulated and downregulated genes in PMF compared to NBM. (CSV 725 kb)
Supplementary Table 4
The upregulated and downregulated genes in PMF compared to PV. (CSV 72 kb)
Supplementary Table 5
The upregulated and downregulated genes in PMF compared to ET. (CSV 71 kb)
Supplementary Table 6
The upregulated and downregulated genes in PV compared to ET. (CSV 236 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, W., Cao, W., Shi, J. et al. Contributions of bone marrow monocytes/macrophages in myeloproliferative neoplasms with JAK2V617F mutation. Ann Hematol 102, 1745–1759 (2023). https://doi.org/10.1007/s00277-023-05284-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05284-5