What are immunoglobulins (types, structure…) 11th October 2022 – Tags: immunoglobulins
Many people may wonder what are immunoglobulins and what types there are, in this article we will answer all the questions about their functions and immunoglobulin structure.
Immunoglobulins, or antibodies, are Y-shaped proteins produced by specialised cells of the immune system called B cells and plasma cells. The immune system produces specific antibodies for each pathogen and can learn to produce specific antibodies for a new virus.
What are immunoglobulins?
Immunoglobulins (Ig) or antibodies are glycoproteins produced by plasma cells. B cells are instructed by specific immunogens, for example bacterial proteins, to differentiate into plasma cells, cells that produce proteins and participate in humoral immune responses against bacteria, viruses, fungi, parasites, cellular antigens, chemicals and synthetic substances.
The immunogen or antigen reacts with a B cell receptor (BCR) on the cell surface of the B cells and a signal is produced that directs the activation of transcription factors to stimulate the synthesis of antibodies, which are highly specific for the immunogen that stimulated the B cell. Furthermore, a B-cell clone is capable of producing proteins that participate in the humoral immune response against bacteria, viruses, fungi, parasites, cellular antigens, chemicals and synthetic substances.
Function of immunoglobulin
Describe the function of immunoglobulins.
Illustrate the disorders associated with immunoglobulin deficiencies.
Summarize the presentation of patients with immunoglobulin deficiency.
Explain the importance of improving care coordination among the interprofessional team to improve the care of immunoglobulin-deficient patients.
Immunoglobulin structure
Immunoglobulin structure is made up of two light chains and two heavy chains in a light-heavy-light-light structure. The heavy chains differ between classes. They have an Fc region that performs biological functions (for example, the ability to bind to cell receptors) and a Fab region that contains antigen-binding sites. The chains are folded into regions called domains. There are four or five domains in the heavy chain, depending on its class, and two domains in the light chain. The hypervariable regions (HRRs) contain the antigen binding sites. All antibodies perform one or more (bifunctional) functions, including activating the complement system, opsonising microbes to facilitate phagocytosis, preventing microbes from attaching to mucosal surfaces and neutralising toxins and viruses.
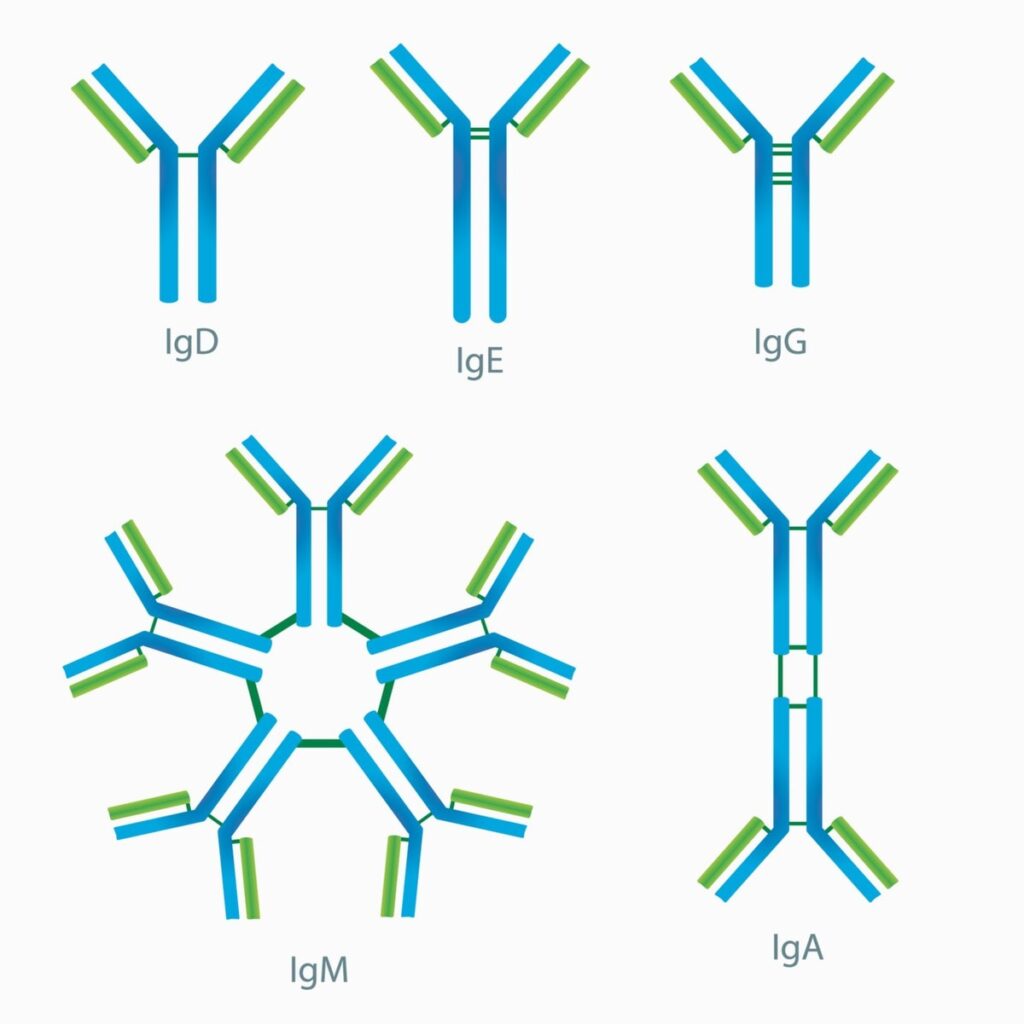

Types of immunoglobulins
The human body produces only five types of immunoglobulins:
- IgG
- IgM
- IgA
- IgD
- IgE
These immunoglobulins protect the body against different types of antigens. All antibodies differ in their amino acid sequence in the constant region, their structure (monomer, pentamer and dimer), their short duration in the blood, their site and their immunological properties.
Immunoglobulin G (IgG)
Immunoglobulin G (IgG) is the most common and abundant antibody in the body.
Blood plasma is composed of 75-80% IgG antibodies.
Of all the antibodies, IgG has the longest life span, about 23 days.
IgG is the only antibody that can cross the placental barrier and confer passive immunity to the developing foetus.
IgG antibodies are reminiscent of pathogens that have previously entered the body and caused infection. IgG also confers some immunity to infants when ingested through breast milk.
The function of IgG is to increase phagocytosis of pathogens, neutralise bacterial or viral toxins and activate the complement system.
IgG has four isotypes:
- IgG1
- IgG2
- IgG3
- IgG4
Immunoglobulin M (IgM)
Immunoglobulin M (IgM) is the first antibody that interacts with new bacteria entering the body and triggers the primary immune response.
IgM is also called a natural antibody because it is the first line of defence in the immune system and provides short-term protection.
IgM has a giant pentameric structure, superior to all other antibodies and consisting of 10 antigen binding sites, which makes it more effective than IgG in killing bacteria or viruses.
The IgM antibody lasts about five days in our bodies and makes up 5-10% of the antibodies in blood plasma.
IgM also causes agglutination (clustering) of bacteria when it binds to their surface epitope. IgM is known as a potent agglutinin and is also found on the surface of naive B cells and red blood cells in its monomeric form.
Immunoglobulin A (IgA)
Immunoglobulin A (IgA) is the most common antibody after IgG. IgA is found in blood, lymph and other body secretions such as saliva, tears and milk. It can also be found in the genital mucosa, respiratory tract and intestinal mucosa.
IgA antibodies protect the body against bacterial growth and colonisation. However, it is less stable than IgG and is found in smaller quantities, accounting for about 10-15% of total immunoglobulins in the blood.
IgA is often called a secretory antibody because it has a secretory component that protects it from enzymatic digestion. Humans secrete about 5-15 g of secretory IgA daily in mucosal secretions to prevent pathogenesis.
Immunoglobulin D (IgD)
Immunoglobulin D (IgD) constitutes less than 0.5% of serum antibodies. IgD is found in smaller amounts in lymphatic fluids and blood. The function of IgD is still unknown, but it is present on the surface of immature B cells as a receptor and is part of the innate immune system. Researchers believe that IgD regulates the activation and differentiation of B cells into plasma cells.
Immunoglobulin E (IgE)
Immunoglobulin E (IgE) constitutes less than 0.01% of serum antibodies. Although it is present in minimal amounts in the blood and has a shorter life span, it is still a potent antibody.
IgE is the most effective antibody against parasitic infections, including helminths.
High levels of IgE in the blood can sometimes cause hypersensitivity to non-harmful substances, such as pollen or dust particles. Recently, immunologists have started to formulate new anti-IgE antibodies for the treatment of allergies and asthma.