Plantago major L., the Greater Plantain (also called Waybread), Plantago lanceolata L., the Ribwort Plantain and Plantago media L., the Hoary Plantain
All these three plantain species are rosette-forming perennials with several leafless inflorescence spikes bearing tiny tubular flowers. Along with Plantago maritima and P. coronopus (see our previous blog) all are native to the British Isles.



From left to right: P. major, P. lanceolata, P. media. Images: ©Chris Jeffree
The maps below show that Plantago major, the greater plantain, and Plantago lanceolata, the ribwort plantain occur in almost every hectad (10 km square) in the UK, while P. media, the hoary plantain, is relatively rare in Scotland. All species grow in pastures and waste places, particularly in anthropogenic habitats. All three are also native throughout Europe, while P. lanceolata and P. major are cosmopolitan, occurring as alien weeds in every continent on Earth, except Antarctica. P. major was brought to the US by European settlers and is thought to have been already well established in the seventeenth century.
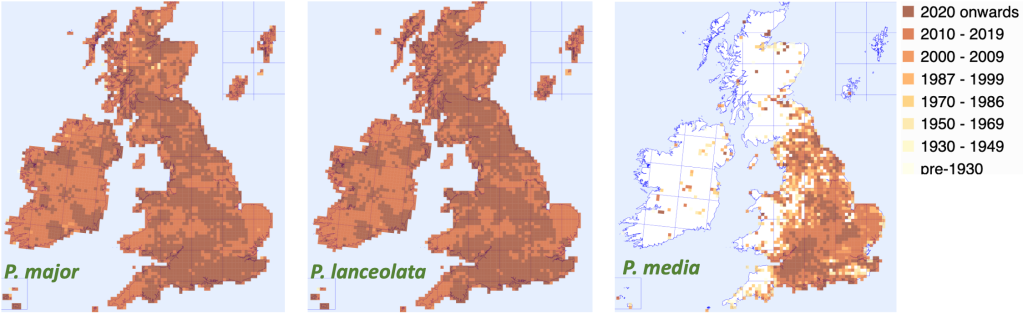
Distribution of the three species in the British Isles. From BSBI/Maps.
In New England indigenous people referred to P. major as “Englishman’s foot” because it thrived on disturbed soil, following colonists wherever they went. In fact, the name Plantago comes from the Latin words ‘planta’, meaning ’sole of the foot’ and ago, meaning ’-like’, together meaning ’like a footprint or sole’. Plantains can certainly be spread by human feet, but it could also be a ‘double entendre’ because hoary plantain leaves could be thought to resemble the shape of a footprint.
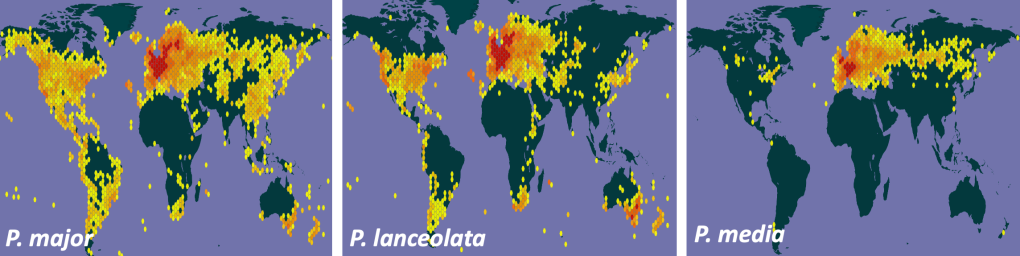
Distribution of the three species in the world, from GBIF.
Plantago major is included in a 10th/11th century herbal ‘Nine Herbs Charm’, which details nine sacred herbs of the Anglo-Saxons – thought to have been given to humans by Woden, the God of healing. In Old English, the plantain is quaintly addressed as ‘wegbraid, wyrta modor’ (Waybread, mother of herbs). The name ‘waybread’ derives from its propensity to grow where the earth is most densely packed, like paths and roadways.
“And you, waybread, mother of herbs,
open from the east, mighty inside.
Over you chariots creaked, over you queens rode,
over you brides cried out, over you bulls snorted.
You withstood all of them, you dashed against them.
May you likewise withstand poison and infection
and the loathsome foe roving through the land.”


Detail of P. major with its longer flower spikes and long petioles. Images: ©Chris Jeffree
The distribution map for P. media indicates that it becomes increasingly rare towards North and West of Scotland. It could also be that it favours higher pH, particularly chalky soils which are rare in Scotland. Alternatively, it may be limited by lower summer temperatures. Its presence in East Lothian and along the River Tweed, where there is a comparatively high level of summer sunshine, might support that explanation. It will be interesting to see whether we will be seeing more of it as the climate warms.


Some details of P. lanceolata. Left, the lance-shaped leaves are quite variable in width. Right, inflorescence of many tubular flowers (note the ridged stalks). Images: ©Chris Jeffree
P. lanceolata and P. major are easily distinguished from one another: P. lanceolata has a ridged flower stem (scape) and shorter flower spikes, whereas P. major has much broader leaves with clearly discernible petioles. P. media, usually distinguished by its short petioles, hoary pubescence and longer filaments, can be confused with P. major, especially in the vegetative state. It is hoary (covered with greyish short hairs) as the name implies, but then P. major can also be pubescent, and its petioles can vary in length. The differences are blurred by the fact that all three species are highly variable, both by nature (genetic differences) and nurture (plasticity).


P. media. Left: note the pinkish-purple flowers and long styles, which make them attractive to insects. The right image shows the short petiole. Left image © C.E. Jeffree; right © R. Milne
Plant identification is further complicated as P. major exists as two subspecies: ssp major and ssp intermedia, the latter of which shares several characteristics with P. media. The two subspecies are said to differ from each other in seed number per capsule (more in ssp intermedia) and vein number per leaf (more in ssp major) (Stace, 1997). They have been known to hybridise (Elbekatoushi, Richards and Wolff, 2007), but despite this some taxonomists would like to elevate them to the rank of species (Morgan- Richards and Wolff, 2002). Both subspecies are well adapted to trampling. Species P. media, P. lanceolata, and P. major never cross to produce hybrids – in fact, all attempts to produce artificial hybrids between them have failed (Sagar and Harper, 1964). These authors concluded that there is an absolute barrier to hybridization between these species. The chromosome numbers in the three species are 2n=24, 2n=12 and 2n=12, respectively – the tetraploid populations of P. media thought to have arisen through autotetraploidy (Van Dijkt and van Delden, 1990).
To clarify, I have attempted to summarise the differences between the taxa in the table below (information gathered from several sources).
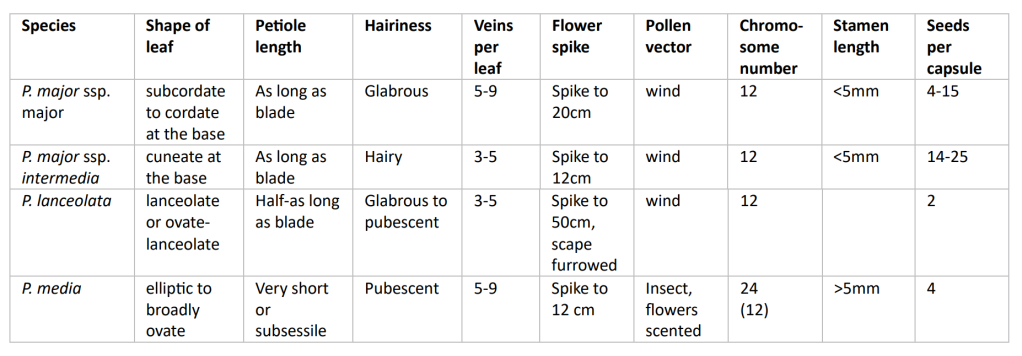
All of the above are self-compatible, and all except P. media are wind pollinated. P. media flowers are in fact pinkish-purple and scented, adapted to attract insects. All have bisexual flowers with their parts in fours. Large plants of P. major have been known to produce up to 30, 000 seeds, which are attractive to birds (Salisbury, 1961). All species show high self-fertilisation rates allowing for the evolution of genotypically and phenotypically distinct local populations. An elegant study of 50 worldwide populations of P. major ssp major, enticingly entitled ‘Travels of a worldwide weed’, identified patterns of genomic diversity which follow colonial trade routes (Ahlstrand et al, 2022).
So, plant populations adapted as they went to local conditions of temperature, soil pH, heavy metal pollution and trampling. In addition, the species are very ‘plastic’, able to adapt to environmental cues during their own lifetime, and this plasticity is itself under genetic control. We have all noticed that plantains can vary their growth form in response to environmental conditions; in shorter grass and mown areas the leaves are shorter and held flat to the ground, while in longer grass the leaves are longer and more angled off the grass. Lotz and Blom (1986) demonstrated phenotypic plasticity in life history traits in Plantago major ssp intermedia populations from different sites. They suggest that phenotypic plasticity expressed in either vegetative or generative development is an important response to selective forces. Miehe-Steier at al (2015) analysed chemical plasticity in P. lanceolata using experiments from greenhouse studies. They found that phenotypic plasticity in response to environmental variation rather than genetic differentiation in response to plant community diversity is responsible for variation in secondary metabolite concentrations of P. lanceolata. Due to its large phenotypic plasticity P. lanceolata has the potential for a fast and efficient adjustment to varying environmental conditions in plant communities of different species richness and functional composition. Similarly, plasticity in spike colour allows P. lanceolata to adapt to different light and temperature regimes, the darker, less reflective spikes absorbing more incoming solar radiation than lighter, more reflective spikes, thereby helping to warm reproductive tissues (Marshall et al, 2019).
All three species can grow in heavily polluted areas, such as mine waste sites and next to roads and have even been said to act as heavy metal accumulators, sequestering cadmium, lead, zinc, copper, iron and manganese in their tissues at levels higher than in the surrounding soil – particularly in their roots (Guzwa-Przepióra et al, 2016). The authors concluded that the species may be considered for the phytostabilization of heavy metal contaminated areas. Muhamedagic and Cero (2020) found P. lanceolata to be particularly effective accumulator of arsenic and cadmium. In another study (Pietrelli, Menegoni and Papetti, 2022), compared 7 herbaceous species, including P. lanceolata, for accumulation of Cd, Cr, Cu, Ni, Pb, and Zn, and of those P. lanceolata was shown to have the highest heavy metal uptake.
Pollution can also affect Plantago species in different, often weird ways. On a lockdown walk in the Edinburgh area, I was amazed to see very peculiar fasciated flower heads on P. lanceolata (see below). Chris Jeffree found different oddities: one with short, sedge-like flowers, and another plant bearing shoots up the flower stem (see below). The Oxford University website suggests the cause as sub-lethal doses of herbicide runoff, while others suggest genetic mutation or viral or bacterial attack. We have no idea what caused the deformations in the examples we found.

Fasciated flower heads of P. lanceolata. Left: photo ©M. Chamberlain; middle and right: photo ©C.E. Jeffree
All three species which are being discussed in this blog have a long history of human use both in native and introduced ranges, but it is clearly best to harvest them from unpolluted sites. Herbalists still use them for wounds, to staunch blood, to pull tissues together or to help expel infection or venom. If you cut a finger while out walking, P. lanceolata, which conveniently comes with strap-like leaves, can be wrapped round the cut like a make-shift plaster.
More recently its medicinal benefits have been subject to scientific studies. Samuelsen (2000) reviewed the ethnopharmacological uses of P. major. I summarise them on the table below.
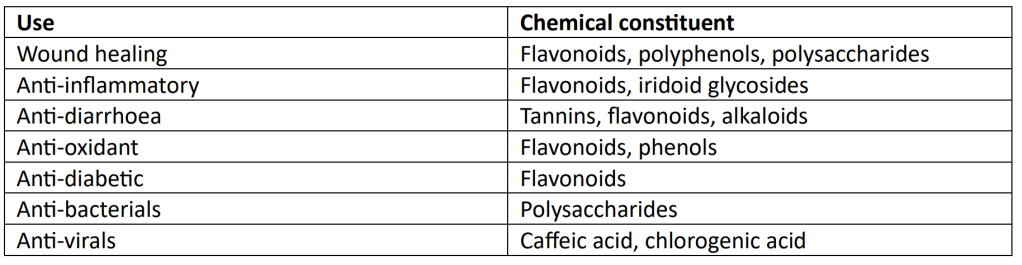
P. lanceolata is equally effective, for the very same uses, but in addition contains cinnamic acid and anthocyanins, which are effective as cytotoxins and for anti-parasite activity. Roots, leaves and flowers are used (Abate et al, 2022). Another source reports the use of the plant as an anti-fertility agent (Pramanik and Rayhaudhuri, 1997). P. media has been less well studied for its potential uses, but a recent study by Fierascu et al (2021) has found it to be equally constituent rich.
One thing that I have not yet mentioned is the ingestion of the three species of plantains by grazing animals. All three are good for cattle – as long as the pasture is not contaminated with heavy metals. The use of P. lanceolata in cattle diet was proven to reduce N2O emissions (Simon et al, 2019) and to increase the growth performance and carcass characteristics of lambs (Somasiri et al, 2015).
The last thing I should mention is that Plantago lanceolata has for centuries been used by children in several inventive games. The flower stems and heads were used like conkers, or the peduncles were looped round the head and the head fired off. In Denmark children pulled of ribwort’s ‘ribs’ one by one, saying ‘She loves me, she loves me not’. I used them more mundanely, stripping off the seeds to make soup for my dolls. Ah for those times – when summers were long, toys were hard to find, and screens had not been thought of.
References
Abate, L., Bachheti, R.K., Tadesse, M.G., and Bachheti, A. (2022). Ethnobotanical uses, chemical constituents and application of Plantago lanceolata L. Journal of Chemistry: Phytochemistry, Ethnopharmacology, and Bioavailability of Medicinal Plants. https://doi.org/10.1155/2022/1532031
Ahlstrand, N.I. et al (2022) Travel Tales of a Worldwide Weed: Genomic Signatures of Plantago major L. Reveal Distinct Genotypic Groups With Links to Colonial Trade Routes. Front. Plant Sci.,13 https://www.frontiersin.org/articles/10.3389/fpls.2022.838166/full
Elbekatoushi, R., Richards, A.J. and Wolff, K. (2007). Introgression between Plantago major L. subspecies major and subspecies intermedia (Gilib.) Lange. in a British population. Watsonia 26, 373-379.
Guzwa-Przepióra, E., Nadgórska-Socha, A., Fojcik, B., Chmura, D. (2016). Enzymatic activities and arbuscular mycorrhizal colonization of Plantago lanceolata and Plantago major in a soil root zone under heavy metal stress. Environmental Science and Pollution Research, 23, 4742–4755
Fierascu RC, Fierascu I, Ortan A, Paunescu A. Plantago media L. (2021) Explored and Potential Applications of an Underutilized Plant. Plants (Basel).10(2), 265.
Lotz, L.A.P. and Blom, C.W.P.M., (1986). Plasticity in life-history traits of Plantago major L. ssp. pleiosperma Pilger (=intermedia Gilib). Oecologia 69, 25-30.
Marshall, M.M., Batten, L.C., Remington, D.L., Lacey, E.P. (2019). Natural selection contributes to geographic patterns of thermal plasticity in Plantago lanceolata. Ecology and Evolution https://doi.org/10.1002/ece3.4977
Miehe-Steier, A., Roscher, C., Reichelt, M., Gershenzon, J., Unsicke, S.B. (2015). Light and nutrient responses in secondary metabolites of Plantago lanceolata offspring are due to phenotypic plasticity in experimental grasslands. Plus One, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0136073
Morgan-Richards, M., and Wolff, K. (2002). Genetic structure and differentiation of Plantago major reveals a pair of sympatric sister species. Mol. Ecol. 8, 1027–1036.
Muhamedagic, F. and Cero, M. (2020). Application of eco-compatible technology – phytoremediation- case study with phytoaccumulator Plantago lanceolata. New Technologies in Agriculture, Ecology, Chemical Processes. See here.
Pietrelli, L., Menegoni, P., Papetti, P. (2022). Bioaccumulation of heavy metals by herbaceous species grown in urban and rural sites. Water, Air and Soil Pollution233, 141.
Pramanik, S. and Rayhaudhuri, S. (1997) DNA Content, Chromosome Composition, and Isozyme Patterns in Plantago L. Botanical Review 63, 124-139.
Salisbury, E., (1961) Weeds and Aliens. Harper Collins Publishers.
Sagar, G.R. and Harper, J.L. (1964). Plantago major L., P. media L. and P. lanceolata L. Journal of Ecology 52, 189-221.
Samuelson, A.B. (2000). The traditional uses, chemical constituents and biological activities of Plantago major L. A review. Journal of Ethnopharmacology, 71, 1-21.
Simon P.L., de Klein C.A.M., Worth W., Rutherford A.J., Dieckow J. (2019). The efficacy of Plantago lanceolata for mitigating nitrous oxide emissions from cattle urine patches. Sci. Total Environ. 691, 430–441.
Somasiri S.C., Kenyon P.R., Kemp P.D., Morel P.C.H., Morris S. (2015).Growth performance and carcass characteristics of lambs grazing forage mixes inclusive of plantain (Plantago lanceolata L.) and chicory (Cichorium intybus L.) Small Rumin. Res. 127, 20–27.
Stace, C. (1997). New Flora of the British Isles. Second edition.Cambridge University Press.
Van Dijkt, P. and van Delden, W. (1990). Evidence for autotetraploidy in Plantago media and comparisons between natural and artificial cytotypes concerning cell size and fertility. Heredity 65, 349-357.
©Maria Chamberlain
Thanks for this great blog post! About the mating system – I think in P. lanceolata, individuals can have either bisexual or female-only flowers (gynodioecy).
LikeLike